Reticular Formation Connections Underlying Horizontal Gaze: The Central Mesencephalic Reticular Formation (cMRF) as a Conduit for the Collicular Saccade Signal
- PMID: 28487639
- PMCID: PMC5403835
- DOI: 10.3389/fnana.2017.00036
Reticular Formation Connections Underlying Horizontal Gaze: The Central Mesencephalic Reticular Formation (cMRF) as a Conduit for the Collicular Saccade Signal
Abstract
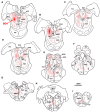
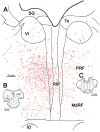
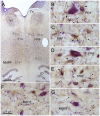
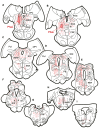
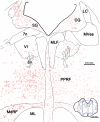
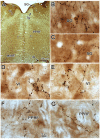
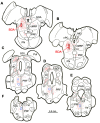
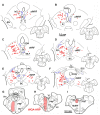
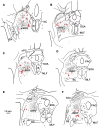
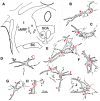
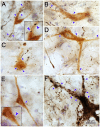
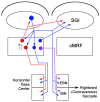
The central mesencephalic reticular formation (cMRF) occupies much of the core of the midbrain tegmentum. Physiological studies indicate that it is involved in controlling gaze changes, particularly horizontal saccades. Anatomically, it receives input from the ipsilateral superior colliculus (SC) and it has downstream projections to the brainstem, including the horizontal gaze center located in the paramedian pontine reticular formation (PPRF). Consequently, it has been hypothesized that the cMRF plays a role in the spatiotemporal transformation needed to convert spatially coded collicular saccade signals into the temporally coded signals utilized by the premotor neurons of the horizontal gaze center. In this study, we used neuroanatomical tracers to examine the patterns of connectivity of the cMRF in macaque monkeys in order to determine whether the circuit organization supports this hypothesis. Since stimulation of the cMRF produces contraversive horizontal saccades and stimulation of the horizontal gaze center produces ipsiversive saccades, this would require an excitatory cMRF projection to the contralateral PPRF. Injections of anterograde tracers into the cMRF did produce labeled terminals within the PPRF. However, the terminations were denser ipsilaterally. Since the PPRF located contralateral to the movement direction is generally considered to be silent during a horizontal saccade, we then tested the hypothesis that this ipsilateral reticuloreticular pathway might be inhibitory. The ultrastructure of ipsilateral terminals was heterogeneous, with some displaying more extensive postsynaptic densities than others. Postembedding immunohistochemistry for gamma-aminobutyric acid (GABA) indicated that only a portion (35%) of these cMRF terminals are GABAergic. Dual tracer experiments were undertaken to determine whether the SC provides input to cMRF reticuloreticular neurons projecting to the ipsilateral pons. Retrogradely labeled reticuloreticular neurons were predominantly distributed in the ipsilateral cMRF. Anterogradely labeled tectal terminals were observed in close association with a portion of these retrogradely labeled reticuloreticular neurons. Taken together, these results suggest that the SC does have connections with reticuloreticular neurons in the cMRF. However, the predominantly excitatory nature of the ipsilateral reticuloreticular projection argues against the hypothesis that this cMRF pathway is solely responsible for producing a spatiotemporal transformation of the collicular saccade signal.
Keywords: PPRF; eye movement; gaze; oculomotor; saccade; superior colliculus.
Figures


Similar articles
-
Anatomical evidence that the superior colliculus controls saccades through central mesencephalic reticular formation gating of omnipause neuron activity.J Neurosci. 2013 Oct 9;33(41):16285-96. doi: 10.1523/JNEUROSCI.2726-11.2013. J Neurosci. 2013. PMID: 24107960 Free PMC article.
-
The feedback circuit connecting the central mesencephalic reticular formation and the superior colliculus in the macaque monkey: tectal connections.Exp Brain Res. 2008 Aug;189(4):485-96. doi: 10.1007/s00221-008-1444-3. Epub 2008 Jun 14. Exp Brain Res. 2008. PMID: 18553075 Free PMC article.
-
The macaque midbrain reticular formation sends side-specific feedback to the superior colliculus.Exp Brain Res. 2010 Apr;201(4):701-17. doi: 10.1007/s00221-009-2090-0. Epub 2009 Nov 26. Exp Brain Res. 2010. PMID: 19940983 Free PMC article.
-
Neuroanatomy of the oculomotor system. The reticular formation.Rev Oculomot Res. 1988;2:119-76. Rev Oculomot Res. 1988. PMID: 3153645 Review.
-
Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts.Ann N Y Acad Sci. 2002 Apr;956:85-98. doi: 10.1111/j.1749-6632.2002.tb02811.x. Ann N Y Acad Sci. 2002. PMID: 11960796 Review.
Cited by
-
Central mesencephalic reticular formation control of the near response: lens accommodation circuits.J Neurophysiol. 2019 May 1;121(5):1692-1703. doi: 10.1152/jn.00846.2018. Epub 2019 Mar 6. J Neurophysiol. 2019. PMID: 30840529 Free PMC article.
-
Is the central mesencephalic reticular formation a purely horizontal gaze center?Brain Struct Funct. 2022 Sep;227(7):2367-2393. doi: 10.1007/s00429-022-02532-8. Epub 2022 Jul 24. Brain Struct Funct. 2022. PMID: 35871423
-
TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson's Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations.Toxics. 2022 Sep 24;10(10):559. doi: 10.3390/toxics10100559. Toxics. 2022. PMID: 36287840 Free PMC article.
-
The Superior Colliculus: Cell Types, Connectivity, and Behavior.Neurosci Bull. 2022 Dec;38(12):1519-1540. doi: 10.1007/s12264-022-00858-1. Epub 2022 Apr 28. Neurosci Bull. 2022. PMID: 35484472 Free PMC article. Review.
-
Saccades evoked in response to electrical stimulation of the posterior bank of the arcuate sulcus.Exp Brain Res. 2017 Sep;235(9):2797-2809. doi: 10.1007/s00221-017-5012-6. Epub 2017 Jun 20. Exp Brain Res. 2017. PMID: 28634888
References
-
- Barnerssoi M., May P. J. (2016). “Postembedding immunohistochemistry for inhibitory neurotransmitters in conjunction with neuroanatomical tracers,” in Transmission Electron Microscopy Methods for Understanding the Brain (Vol. 115), ed. Van Bockstaele E. J. (New York, NY: Springer; ), 181–203.
-
- Bender M. B., Shanzer S. (1964). “Oculomotor pathways defined by electrical stimulation and lesion in the brainstem of the monkey,” in The Oculomotor System, ed. Bender M. B. (New York, NY: Harper and Row; ), 81–140.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

