Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative
- PMID: 28487651
- PMCID: PMC5403950
- DOI: 10.3389/fphar.2017.00205
Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative
Abstract
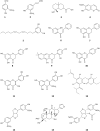
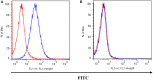
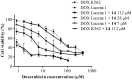
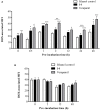
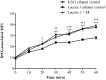
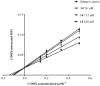
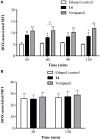
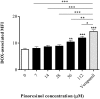
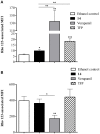
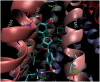
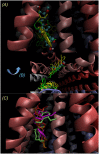
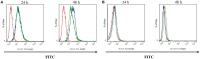
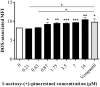
P-glycoprotein (P-gp) is a membrane protein associated with multidrug resistance (MDR) due to its key role in mediating the traffic of chemotherapeutic drugs outside cancer cells, leading to a cellular response that hinders efforts toward successful therapy. With the aim of finding agents that circumvent the MDR phenotype mediated by P-gp, 15 compounds isolated from native and naturalized plants of Argentina were screened. Among these, the non-cytotoxic lignan (±) pinoresinol successfully restored sensitivity to doxorubicin from 7 μM in the P-gp overexpressed human myelogenous leukemia cells, Lucena 1. This resistance-reversing effect was confirmed by competitively increasing the intracellular doxorubicin accumulation and by significantly inhibiting the efflux of doxorubicin and, to a lesser extent, that of rhodamine 123. The activity obtained was similar to that observed with verapamil. No such results were observed in the sensitive parental K562 cell line. To gain deeper insight into the mode of action of pinoresinol, its effect on P-gp function and expression was examined. The docking simulations indicated that the lignan bound to P-gp at the apex of the V-shaped transmembrane cavity, involving transmembrane helices 4, 5, and 6, and partially overlapped the binding region of tariquidar, which was used as a positive control. These results would shed some light on the nature of its interaction with P-gp at molecular level and merit further mechanistic and kinetic studies. In addition, it showed a maximum 29% activation of ATP hydrolysis and antagonized verapamil-stimulated ATPase activity with an IC50 of 20.9 μM. On the other hand, pinoresinol decreased the presence of P-gp in the cell surface. Derivatives of pinoresinol with improved activity were identified by docking studies. The most promising one, the non-cytotoxic 1-acetoxypinoresinol, caused a reversion of doxorubicin resistance from 0.11 μM and thus higher activity than the lead compound. It also caused a significant increase in doxorubicin accumulation. Results were similar to those observed with verapamil. The results obtained positioned these compounds as potential candidates for effective agents to overcome P-gp-mediated MDR, leading to better outcomes for leukemia chemotherapy.
Keywords: (±) pinoresinol; 1-acetoxy-(+)-pinoresinol; P-glycoprotein; multidrug resistance reversal; plant-derived compounds.
Figures

References
-
- Alonso J., Desmarchelier C. (2015). Plantas Medicinales Autóctonas de la Argentina: Bases cientÍficas Para su Aplicación en Atención Primaria de la Salud. Buenos Aires: Corpus Editorial y Distribuidora.
-
- Carpinella M. C., De Bellis L., Joray M. B., Sosa V., Zunino P. M., Palacios S. M. (2011). Inhibition of development, swarming differentiation and virulence factors in Proteus mirabilis by an extract of Lithrea molleoides and its active principle (Z,Z)-5-(trideca-4',7'-dienyl)-resorcinol. Phytomedicine 18, 994–997. 10.1016/j.phymed.2011.03.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

