Functional Gene Analysis Reveals Cell Cycle Changes and Inflammation in Endothelial Cells Irradiated with a Single X-ray Dose
- PMID: 28487652
- PMCID: PMC5404649
- DOI: 10.3389/fphar.2017.00213
Functional Gene Analysis Reveals Cell Cycle Changes and Inflammation in Endothelial Cells Irradiated with a Single X-ray Dose
Abstract
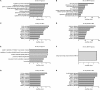
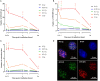
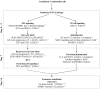
Background and Purpose: Epidemiological data suggests an excess risk of cardiovascular disease (CVD) at low doses (0.05 and 0.1 Gy) of ionizing radiation (IR). Furthermore, the underlying biological and molecular mechanisms of radiation-induced CVD are still unclear. Because damage to the endothelium could be critical in IR-related CVD, this study aimed to identify the effects of radiation on immortalized endothelial cells in the context of atherosclerosis. Material and Methods: Microarrays and RT-qPCR were used to compare the response of endothelial cells irradiated with a single X-ray dose (0.05, 0.1, 0.5, 2 Gy) measured after various post-irradiation (repair) times (1 day, 7 days, 14 days). To consolidate and mechanistically support the endothelial cell response to X-ray exposure identified via microarray analysis, DNA repair signaling (γH2AX/TP53BP1-foci quantification), cell cycle progression (BrdU/7AAD flow cytometric analysis), cellular senescence (β-galactosidase assay with CPRG and IGFBP7 quantification) and pro-inflammatory status (IL6 and CCL2) was assessed. Results: Microarray results indicated persistent changes in cell cycle progression and inflammation. Cells underwent G1 arrest in a dose-dependent manner after high doses (0.5 and 2 Gy), which was compensated by increased proliferation after 1 week and almost normalized after 2 weeks. However, at this point irradiated cells showed an increased β-Gal activity and IGFBP7 secretion, indicative of premature senescence. The production of pro-inflammatory cytokines IL6 and CCL2 was increased at early time points. Conclusions: IR induces pro-atherosclerotic processes in endothelial cells in a dose-dependent manner. These findings give an incentive for further research on the shape of the dose-response curve, as we show that even low doses of IR can induce premature endothelial senescence at later time points. Furthermore, our findings on the time- and dose-dependent response regarding differentially expressed genes, cell cycle progression, inflammation and senescence bring novel insights into the underlying molecular mechanisms of the endothelial response to X-ray radiation. This may in turn lead to the development of risk-reducing strategies to prevent IR-induced CVD, such as the use of cell cycle modulators and anti-inflammatory drugs as radioprotectors and/or radiation mitigators.
Keywords: X-ray; atherosclerosis; cardiovascular disease; cell cycle; endothelium.
Figures


Similar articles
-
In vitro Assessment of the DNA Damage Response in Dental Mesenchymal Stromal Cells Following Low Dose X-ray Exposure.Front Public Health. 2021 Feb 15;9:584484. doi: 10.3389/fpubh.2021.584484. eCollection 2021. Front Public Health. 2021. PMID: 33692980 Free PMC article.
-
Residual γH2AX foci induced by low dose x-ray radiation in bone marrow mesenchymal stem cells do not cause accelerated senescence in the progeny of irradiated cells.Aging (Albany NY). 2017 Nov 21;9(11):2397-2410. doi: 10.18632/aging.101327. Aging (Albany NY). 2017. PMID: 29165316 Free PMC article.
-
Differential Impact of Single-Dose Fe Ion and X-Ray Irradiation on Endothelial Cell Transcriptomic and Proteomic Responses.Front Pharmacol. 2017 Sep 22;8:570. doi: 10.3389/fphar.2017.00570. eCollection 2017. Front Pharmacol. 2017. PMID: 28993729 Free PMC article.
-
DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases.J Radiat Res. 2021 Jul 10;62(4):564-573. doi: 10.1093/jrr/rrab032. J Radiat Res. 2021. PMID: 33912932 Free PMC article. Review.
-
Radiation-Induced Bystander Response: Mechanism and Clinical Implications.Adv Wound Care (New Rochelle). 2014 Jan 1;3(1):16-24. doi: 10.1089/wound.2013.0468. Adv Wound Care (New Rochelle). 2014. PMID: 24761341 Free PMC article. Review.
Cited by
-
Radiation Response of Human Cardiac Endothelial Cells Reveals a Central Role of the cGAS-STING Pathway in the Development of Inflammation.Proteomes. 2020 Oct 26;8(4):30. doi: 10.3390/proteomes8040030. Proteomes. 2020. PMID: 33114474 Free PMC article.
-
Multiple exposures to low-dose ionizing radiation induced the initiation and progression of pro-atherosclerotic phenotypes in mice and vascular endothelial cell damage.Sci Prog. 2024 Jan-Mar;107(1):368504241228668. doi: 10.1177/00368504241228668. Sci Prog. 2024. PMID: 38385346 Free PMC article.
-
Connexin43 Hemichannel Targeting With TAT-Gap19 Alleviates Radiation-Induced Endothelial Cell Damage.Front Pharmacol. 2020 Mar 5;11:212. doi: 10.3389/fphar.2020.00212. eCollection 2020. Front Pharmacol. 2020. PMID: 32210810 Free PMC article.
-
Radiation-Induced Gene Expression Changes in High and Low Grade Breast Cancer Cell Types.Int J Mol Sci. 2018 Apr 4;19(4):1084. doi: 10.3390/ijms19041084. Int J Mol Sci. 2018. PMID: 29617354 Free PMC article.
-
An emerging double‑edged sword role of ferroptosis in cardiovascular disease (Review).Int J Mol Med. 2025 Jan;55(1):16. doi: 10.3892/ijmm.2024.5457. Epub 2024 Nov 14. Int J Mol Med. 2025. PMID: 39540363 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

