RhoA inhibits apoptosis and increases proliferation of cultured SPCA1 lung cancer cells
- PMID: 28487954
- PMCID: PMC5436147
- DOI: 10.3892/mmr.2017.6545
RhoA inhibits apoptosis and increases proliferation of cultured SPCA1 lung cancer cells
Abstract
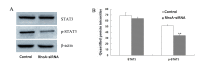
The Rho kinase pathway has previously been reported to possess a close relationship with the growth, migration and invasion of lung cancer cells. However, the molecular mechanisms underlying the effects of this pathway on lung cancer cells are still elusive. The aim of the present study was to investigate the effects and underlying molecular mechanisms of Ras homolog family member A (RhoA) on the proliferation and apoptosis of SPCA1 lung carcinoma cells. Stable SPCA1 lung cancer cell lines, in which RhoA expression was silenced by small interfering RNA, were isolated following Geneticin screening. Inhibition of RhoA expression significantly decreased the proliferation of SPCA1 lung cancer cells, whereas apoptosis was significantly increased (P<0.01) as determined by the MTS tetrazolium assay and flow cytometry analysis, respectively. At the molecular level, knockdown of RhoA resulted in the significant activation of caspase‑3 (P<0.01), and a significant reduction in the levels of phosphorylated signal transducer and activator of transcription (phospho‑STAT3; P<0.01), as determined by western blotting. The results suggested that RhoA knockdown prevents cell proliferation and induces apoptosis in SPCA1 lung cancer cells. Furthermore, the underlying mechanisms responsible for these effects may include the activation of caspase‑3 and the reduction of phospho‑STAT3 levels.
Figures




Similar articles
-
Loss of RhoA expression prevents proliferation and metastasis of SPCA1 lung cancer cells in vitro.Biomed Pharmacother. 2015 Feb;69:361-6. doi: 10.1016/j.biopha.2014.12.004. Epub 2014 Dec 12. Biomed Pharmacother. 2015. PMID: 25661383
-
Upregulation of HIF-1α protects neuroblastoma cells from hypoxia-induced apoptosis in a RhoA-dependent manner.Mol Med Rep. 2015 Nov;12(5):7123-31. doi: 10.3892/mmr.2015.4267. Epub 2015 Aug 28. Mol Med Rep. 2015. PMID: 26323527
-
Overexpression of microRNA‑125a‑3p effectively inhibits the cell growth and invasion of lung cancer cells by regulating the mouse double minute 2 homolog/p53 signaling pathway.Mol Med Rep. 2015 Oct;12(4):5482-6. doi: 10.3892/mmr.2015.4038. Epub 2015 Jul 3. Mol Med Rep. 2015. PMID: 26151241
-
The Rho-kinase inhibitor inhibits proliferation and metastasis of small cell lung cancer.Biomed Pharmacother. 2012 Apr;66(3):221-7. doi: 10.1016/j.biopha.2011.11.011. Epub 2011 Dec 28. Biomed Pharmacother. 2012. PMID: 22425182
-
MiRNA-125a-3p is a negative regulator of the RhoA-actomyosin pathway in A549 cells.Int J Oncol. 2013 May;42(5):1734-42. doi: 10.3892/ijo.2013.1861. Epub 2013 Mar 21. Int J Oncol. 2013. PMID: 23525486
Cited by
-
Hypoxia-Induced FAM13A Regulates the Proliferation and Metastasis of Non-Small Cell Lung Cancer Cells.Int J Mol Sci. 2021 Apr 21;22(9):4302. doi: 10.3390/ijms22094302. Int J Mol Sci. 2021. PMID: 33919074 Free PMC article.
-
Transcriptome Sequencing Reveals the Differentially Expressed lncRNAs and mRNAs Involved in Cryoinjuries in Frozen-Thawed Giant Panda (Ailuropoda melanoleuca) Sperm.Int J Mol Sci. 2018 Oct 8;19(10):3066. doi: 10.3390/ijms19103066. Int J Mol Sci. 2018. PMID: 30297640 Free PMC article.
-
miR-152/TNS1 axis inhibits non-small cell lung cancer progression through Akt/mTOR/RhoA pathway.Biosci Rep. 2021 Jan 29;41(1):BSR20201539. doi: 10.1042/BSR20201539. Biosci Rep. 2021. PMID: 33269380 Free PMC article.
-
The Association Between Heat-Shock Protein Polymorphisms and Prognosis in Lung Cancer Patients Treated With Platinum-Based Chemotherapy.Front Pharmacol. 2020 Jul 21;11:1029. doi: 10.3389/fphar.2020.01029. eCollection 2020. Front Pharmacol. 2020. PMID: 32848724 Free PMC article.
-
The Molecular Mechanism of Multiple Organ Dysfunction and Targeted Intervention of COVID-19 Based on Time-Order Transcriptomic Analysis.Front Immunol. 2021 Aug 24;12:729776. doi: 10.3389/fimmu.2021.729776. eCollection 2021. Front Immunol. 2021. PMID: 34504502 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

