Chemokine (C‑C motif) ligand 21/C‑C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial‑mesenchymal transition via the extracellular signal‑regulated kinase signaling pathway
- PMID: 28487957
- PMCID: PMC5436267
- DOI: 10.3892/mmr.2017.6534
Chemokine (C‑C motif) ligand 21/C‑C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial‑mesenchymal transition via the extracellular signal‑regulated kinase signaling pathway
Abstract
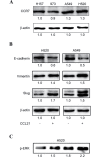
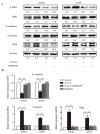
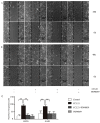
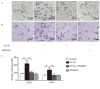
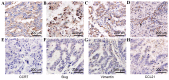
C-C chemokine receptor type 7 (CCR7) has been implicated in lymph node metastasis of various cancers. Previous studies have revealed that epithelial‑mesenchymal transition (EMT) is involved in the chemotactic process mediated by CCR7 and its ligands in various types of carcinoma. However, the underlying mechanism of this process remains to be fully elucidated. The present study investigated whether chemokine (C‑C motif) ligand 21 (CCL21)/CCR7 may activate EMT of lung cancer cells and their associated signaling pathways. A549 and H520 lung cancer cell lines were examined in vitro in the present study. The results indicated that A549 and H520 expressed CCR7, but reduced levels of CCL21. Following stimulation of lung cancer cell lines with CCL21, the expression of the epithelial marker E‑cadherin was downregulated, and the mesenchymal markers Vimentin/Slug and extracellular signal‑regulated kinase (ERK) were upregulated. In addition, the ERK inhibitor PD98059 may inhibit EMT caused by CCL21, and decreased cell migration and invasion initiated by CCL21. Furthermore, lung adenocarcinoma tissues from 50 patients who underwent lung cancer operations were investigated by immunohistochemistry. The findings revealed that CCR7, Slug and Vimentin were highly expressed in lung carcinoma tissues, and were significantly associated with lymph node metastasis and clinical pathological stages, respectively. CCR7 expression was correlated positively with expression levels of Slug and Vimentin. CCL21 was expressed positively in the endothelium of lymphatic vessels adjacent to cancer cells, and weakly in lung cancer cells. Collectively, these results demonstrated that CCL21/CCR7 may activate EMT in lung cancer cells via the ERK1/2 signaling pathway. The current study provides evidence that a close interaction exists between CCL21/CCR7chemotaxis and EMT procedures in lung cancer metastasis, providing a basis for the development of therapeutic targets.
Figures
Similar articles
-
CCL21/CCR7 Axis Contributed to CD133+ Pancreatic Cancer Stem-Like Cell Metastasis via EMT and Erk/NF-κB Pathway.PLoS One. 2016 Aug 9;11(8):e0158529. doi: 10.1371/journal.pone.0158529. eCollection 2016. PLoS One. 2016. PMID: 27505247 Free PMC article.
-
CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells.Int J Clin Exp Pathol. 2015 Dec 1;8(12):15729-38. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26884842 Free PMC article.
-
CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma.Med Oncol. 2014 Sep;31(9):180. doi: 10.1007/s12032-014-0180-8. Epub 2014 Aug 21. Med Oncol. 2014. PMID: 25142946
-
Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes.J Leukoc Biol. 2016 Jun;99(6):869-82. doi: 10.1189/jlb.2MR0815-380R. Epub 2016 Jan 4. J Leukoc Biol. 2016. PMID: 26729814 Review.
-
[Progress in targeting therapy of cancer metastasis by CCL21/CCR7 axis].Sheng Wu Gong Cheng Xue Bao. 2020 Dec 25;36(12):2741-2754. doi: 10.13345/j.cjb.200174. Sheng Wu Gong Cheng Xue Bao. 2020. PMID: 33398969 Review. Chinese.
Cited by
-
Meta-analysis of the prognostic value of C-C chemokine receptor type 7 in patients with solid tumors.Cancer Manag Res. 2019 Feb 26;11:1881-1892. doi: 10.2147/CMAR.S190510. eCollection 2019. Cancer Manag Res. 2019. PMID: 30881115 Free PMC article. Review.
-
Inflammatory markers in women with reported benign gynecologic pathology: an analysis of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.Ann Epidemiol. 2022 Apr;68:1-8. doi: 10.1016/j.annepidem.2021.12.003. Epub 2021 Dec 11. Ann Epidemiol. 2022. PMID: 34906633 Free PMC article.
-
Target organ expression and biomarker characterization of chemokine CCL21 in systemic sclerosis associated pulmonary arterial hypertension.Front Immunol. 2022 Sep 23;13:991743. doi: 10.3389/fimmu.2022.991743. eCollection 2022. Front Immunol. 2022. PMID: 36211384 Free PMC article.
-
C-C Chemokine Receptor 7 in Cancer.Cells. 2022 Feb 14;11(4):656. doi: 10.3390/cells11040656. Cells. 2022. PMID: 35203305 Free PMC article. Review.
-
SNX10 and PTGDS are associated with the progression and prognosis of cervical squamous cell carcinoma.BMC Cancer. 2021 Jun 11;21(1):694. doi: 10.1186/s12885-021-08212-w. BMC Cancer. 2021. PMID: 34116656 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

