Induction of cell cycle arrest by increasing GTP‑RhoA levels via Taxol‑induced microtubule polymerization in renal cell carcinoma
- PMID: 28487984
- PMCID: PMC5436224
- DOI: 10.3892/mmr.2017.6543
Induction of cell cycle arrest by increasing GTP‑RhoA levels via Taxol‑induced microtubule polymerization in renal cell carcinoma
Abstract
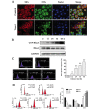
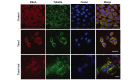
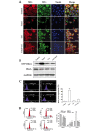
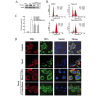
Renal cell carcinoma (RCC) is the most common neoplasm of the kidney in adults, accounting for ~3% of adult malignancies. Understanding the underlying mechanism of RCC tumorigenesis is necessary to improve patient survival. The present study revealed that Taxol‑induced microtubule (MT) polymerization causes cell cycle arrest and an increase in guanosine triphosphate‑Ras homology gene family, member A (GTP‑RhoA) protein expression. Disruption of Taxol‑induced MT polymerization reversed GTP‑RhoA expression and cell cycle arrest. The localization and redistribution of MTs and RhoA were consistent in cells with MT bundles and those without. Decreased GTP‑RhoA had no marked effect on Taxol‑induced MT bundling, however, it reduced the proportion of cells in G2/M phase. Taken together, Taxol‑induced MT polymerization regulated the protein expression levels of GTP‑RhoA and cell cycle arrest. However, the alteration in GTP‑RhoA expression did not influence MT arrangement, suggesting that GTP‑RhoA serves a pivotal role in Taxol‑induced MT polymerization and cell cycle arrest in RCC.
Figures
Similar articles
-
Induction of cell cycle arrest, DNA damage, and apoptosis by nimbolide in human renal cell carcinoma cells.Tumour Biol. 2015 Sep;36(10):7539-47. doi: 10.1007/s13277-015-3477-0. Epub 2015 Apr 28. Tumour Biol. 2015. PMID: 25916210
-
Docetaxel enhances apoptosis and G2/M cell cycle arrest by suppressing mitogen-activated protein kinase signaling in human renal clear cell carcinoma.Genet Mol Res. 2016 Feb 5;15(1). doi: 10.4238/gmr.15017321. Genet Mol Res. 2016. PMID: 26909952
-
Podophyllotoxin acetate triggers anticancer effects against non-small cell lung cancer cells by promoting cell death via cell cycle arrest, ER stress and autophagy.Int J Oncol. 2015 Oct;47(4):1257-65. doi: 10.3892/ijo.2015.3123. Epub 2015 Aug 13. Int J Oncol. 2015. PMID: 26314270 Free PMC article.
-
Recent developments in tubulin polymerization inhibitors: An overview.Eur J Med Chem. 2014 Nov 24;87:89-124. doi: 10.1016/j.ejmech.2014.09.051. Epub 2014 Sep 16. Eur J Med Chem. 2014. PMID: 25240869 Review.
-
Taxol (paclitaxel): mechanisms of action.Ann Oncol. 1994;5 Suppl 6:S3-6. Ann Oncol. 1994. PMID: 7865431 Review.
Cited by
-
Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment.Nat Commun. 2023 Aug 15;14(1):4934. doi: 10.1038/s41467-023-40579-1. Nat Commun. 2023. PMID: 37582846 Free PMC article.
-
Successful first-line treatment of simultaneous multiple primary malignancies of lung adenocarcinoma and renal clear cell carcinoma: A case report.Front Immunol. 2022 Aug 1;13:956519. doi: 10.3389/fimmu.2022.956519. eCollection 2022. Front Immunol. 2022. PMID: 35979370 Free PMC article.
References
-
- Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

