Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes
- PMID: 28487991
- PMCID: PMC5428967
- DOI: 10.3892/ijmm.2017.2967
Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes
Abstract
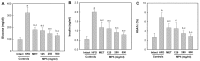
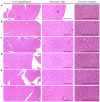
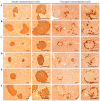
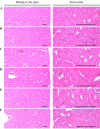
Freshwater animal proteins have long been used as nutrient supplements. In this study, melanian snail (Semisulcospira libertina) protein hydrolysates (MPh) were found to exert anti-diabetic and protective effects against liver and kidney damage in mice with type II diabetes adapted to a 45% kcal high-fat diet (HFD). The hypoglycemic, hepatoprotective and nephroprotective effects of MPh were analyzed after 12 weeks of the continuous oral administration of MPh at 125, 250 and 500 mg/kg. Diabetic control mice exhibited an increase in body weight, and blood glucose and insulin levels, with a decrease in serum high-density lipoprotein (HDL) levels. In addition, an increase in the regions of steatohepatitis, hepatocyte hypertrophy, and lipid droplet deposit-related renal tubular vacuolation degenerative lesions were detected, with noticeable expansion and hyperplasia of the pancreatic islets, and an increase in glucagon- and insulin-producing cells, insulin/glucagon cell ratios in the endocrine pancreas and hepatic lipid peroxidation, as well as decreased zymogen contents. Furthermore, a deterioration of the endogenous antioxidant defense system was observed, with reduced glucose utilization related hepatic glucokinase (GK) activity and an increase in hepatic gluconeogenesis-related phosphoenolpyruvate carboxykinase (PEPCK) and glucose‑6-phosphatase (G6pase) activity. However, all of these diabetic complications were significantly inhibited by oral treatment with MPh in a dose-dependent manner. In addition, the marked dose-dependent inhibition of hepatic lipid peroxidation, the depletion of the liver endogenous antioxidant defense system, and changes in hepatic glucose-regulating enzyme activities were also observed. The results of this study suggest that MPh exerts potent anti-diabetic effects, along with the amelioration of related complications in mice with type II diabetes. The overall effects of MPh at a dose of 125 mg/kg on HFD-induced diabetes and related complications were similar or more potent than those of metformin (250 mg/kg).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

