Platelets as crucial partners for tumor metastasis: from mechanistic aspects to pharmacological targeting
- PMID: 28488110
- PMCID: PMC11107532
- DOI: 10.1007/s00018-017-2536-7
Platelets as crucial partners for tumor metastasis: from mechanistic aspects to pharmacological targeting
Abstract
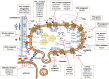
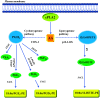
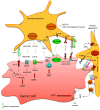
Platelets are anucleated cells that circulate in the blood as sentinels of tissue integrity. In fact, they are rich in a plethora of proteins and other factors stored in different granules which they selectively release upon stimulation. Moreover, platelets synthesize a vast number of lipids and release various types of vesicles, including exosomes which are rich in genetic material. Platelets possess a central function to interact with other cell types, including inflammatory cells and cancer cells. Recent findings have enlightened the capacity of platelets to induce changes in the phenotype of cancer cells which acquire invasiveness thus enhancing their metastatic potential. Thus, it has been hypothesized that targeting the platelet may represent a novel strategy to prevent the development and progression of cancer. This is supported by the efficacy of the antiplatelet agent low-dose aspirin. Studies are ongoing to verify whether other antiplatelet agents share the anticancer effectiveness of aspirin.
Keywords: Antiplatelet agents; Aspirin; Cancer cells; Epithelial–mesenchymal transitions; Metastasis; Platelets; Prostaglandin E2.
Figures
References
-
- World Health Organization (2009) WHO Report on the Global Tobacco Epidemic. Geneva, ISBN: 978 92 4 156391 8
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

