Potential coordination role between O-GlcNAcylation and epigenetics
- PMID: 28488246
- PMCID: PMC5636747
- DOI: 10.1007/s13238-017-0416-4
Potential coordination role between O-GlcNAcylation and epigenetics
Abstract
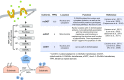
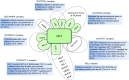
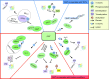
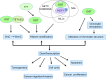
Dynamic changes of the post-translational O-GlcNAc modification (O-GlcNAcylation) are controlled by O-linked β-N-acetylglucosamine (O-GlcNAc) transferase (OGT) and the glycoside hydrolase O-GlcNAcase (OGA) in cells. O-GlcNAcylation often occurs on serine (Ser) and threonine (Thr) residues of the specific substrate proteins via the addition of O-GlcNAc group by OGT. It has been known that O-GlcNAcylation is not only involved in many fundamental cellular processes, but also plays an important role in cancer development through various mechanisms. Recently, accumulating data reveal that O-GlcNAcylation at histones or non-histone proteins can lead to the start of the subsequent biological processes, suggesting that O-GlcNAcylation as 'protein code' or 'histone code' may provide recognition platforms or executive instructions for subsequent recruitment of proteins to carry out the specific functions. In this review, we summarize the interaction of O-GlcNAcylation and epigenetic changes, introduce recent research findings that link crosstalk between O-GlcNAcylation and epigenetic changes, and speculate on the potential coordination role of O-GlcNAcylation with epigenetic changes in intracellular biological processes.
Keywords: O-GlcNAcylation; epigenetics; histone modification; post-translational modification.
Figures
References
-
- Burén S, Gomes AL, Teijeiro A, Fawal MA, Yilmaz M, Tummala KS, Perez M, Rodriguez-Justo M, Campos-Olivas R, Megías D, Djouder N. Regulation of OGT by URI in response to glucose confers c-MYC-dependent survival mechanisms. Cancer Cell. 2016;30:290–307. doi: 10.1016/j.ccell.2016.06.023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

