Impaired swim bladder inflation in early life stage fathead minnows exposed to a deiodinase inhibitor, iopanoic acid
- PMID: 28488362
- PMCID: PMC5733732
- DOI: 10.1002/etc.3855
Impaired swim bladder inflation in early life stage fathead minnows exposed to a deiodinase inhibitor, iopanoic acid
Abstract
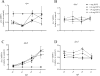
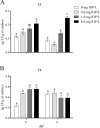
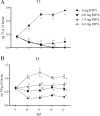
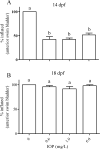
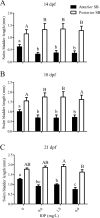
Inflation of the posterior and/or anterior swim bladder is a process previously demonstrated to be regulated by thyroid hormones. We investigated whether inhibition of deiodinases, which convert thyroxine (T4) to the more biologically active form, 3,5,3'-triiodothyronine (T3), would impact swim bladder inflation. Two experiments were conducted using a model deiodinase inhibitor, iopanoic acid (IOP). First, fathead minnow embryos were exposed to 0.6, 1.9, or 6.0 mg/L or control water until 6 d postfertilization (dpf), at which time posterior swim bladder inflation was assessed. To examine anterior swim bladder inflation, a second study was conducted with 6-dpf larvae exposed to the same IOP concentrations until 21 dpf. Fish from both studies were sampled for T4/T3 measurements and gene transcription analyses. Incidence and length of inflated posterior swim bladders were significantly reduced in the 6.0 mg/L treatment at 6 dpf. Incidence of inflation and length of anterior swim bladder were significantly reduced in all IOP treatments at 14 dpf, but inflation recovered by 18 dpf. Throughout the larval study, whole-body T4 concentrations increased and T3 concentrations decreased in all IOP treatments. Consistent with hypothesized compensatory responses, deiodinase-2 messenger ribonucleic acid (mRNA) was up-regulated in the larval study, and thyroperoxidase mRNA was down-regulated in all IOP treatments in both studies. These results support the hypothesized adverse outcome pathways linking inhibition of deiodinase activity to impaired swim bladder inflation. Environ Toxicol Chem 2017;36:2942-2952. Published 2017 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
Keywords: Adverse outcome pathway; Aquatic toxicology; Developmental toxicity; Endocrine-disrupting compounds; Thyroid disruption.
Published 2017 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
Figures


References
-
- Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson BT, Einarsdottir IE, Canario AV, Sweeney GE. Thyroid hormones in growth and development of fish. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:447–459. - PubMed
-
- Lui YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70:36–45. - PubMed
-
- Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA. The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinol. 1997;138:2989–2997. - PubMed
-
- Carr JA, Patino R. The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: Endocrine disruption and its consequences to natural populations. Gen Comp Endocr. 2011;170:299–312. - PubMed
-
- Opitz R, Braunbeck T, Bogi C, Pickford DB, Nentwig G, Oehlmann J, Tooi O, Lutz I, Kloas W. Description and initial evaluation of a Xenopus metamorphosis assay for detection of thyroid system-disrupting activities of environmental compounds. Environ Toxicol Chem. 2005;24:653–664. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

