Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells
- PMID: 28488695
- PMCID: PMC5436221
- DOI: 10.1038/ncomms15207
Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells
Retraction in
-
Retraction Note: Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells.Nat Commun. 2025 Oct 7;16(1):8888. doi: 10.1038/s41467-025-64654-x. Nat Commun. 2025. PMID: 41057331 Free PMC article. No abstract available.
Abstract
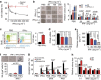
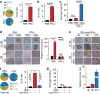
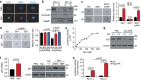
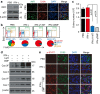
Interactions with the immune system may lead tumorigenic cells into dormancy. However, the underlying molecular mechanism is poorly understood. Using a 3D fibrin gel model, we show that IFN-γ induces tumour-repopulating cells (TRCs) to enter dormancy through an indolamine 2,3-dioxygenase 1 (IDO1)-kynurenine (Kyn)-aryl hydrocarbon receptor (AhR)-p27 dependent pathway. Mechanistically, IFN-γ signalling triggers differentiated tumour cell apoptosis via STAT1; however, when IDO1 and AhR are highly expressed as in TRCs, IFN-γ results in IDO1/AhR-dependent p27 induction that prevents STAT1 signalling, thus suppressing the process of cell death and activating the dormancy program. Blocking the IDO/AhR metabolic circuitry not only abrogates IFN-γ-induced dormancy but also results in enhanced repression of tumour growth by IFN-γ-induced apoptosis of TRCs both in vitro and in vivo. These data present a previously unrecognized mechanism of inducing TRC dormancy by IFN-γ, suggesting a potential effective cancer immunotherapeutic modality through the combination of IFN-γ and IDO/AhR inhibitors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- MacKie R. M., Reid R. & Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N. Engl. J. Med. 348, 567–568 (2003). - PubMed
-
- Milton C. A. et al. The transmission of donor-derived malignant melanoma to a renal allograft recipient. Clin. Transplant. 20, 547–550 (2006). - PubMed
-
- Strauss D. C. & Thomas J. M. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 11, 790–796 (2010). - PubMed
-
- Senzer N. N. et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 27, 5763–5771 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

