Senescent tumor cells lead the collective invasion in thyroid cancer
- PMID: 28489070
- PMCID: PMC5436223
- DOI: 10.1038/ncomms15208
Senescent tumor cells lead the collective invasion in thyroid cancer
Abstract
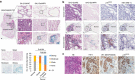
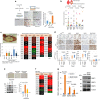
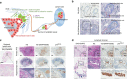
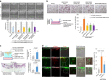
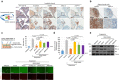
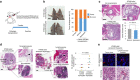
Cellular senescence has been perceived as a barrier against carcinogenesis. However, the senescence-associated secretory phenotype (SASP) of senescent cells can promote tumorigenesis. Here, we show senescent tumour cells are frequently present in the front region of collective invasion of papillary thyroid carcinoma (PTC), as well as lymphatic channels and metastatic foci of lymph nodes. In in vitro invasion analysis, senescent tumour cells exhibit high invasion ability as compared with non-senescent tumour cells through SASP expression. Collective invasion in PTC is led by senescent tumour cells characterized by generation of a C-X-C-motif ligand (CXCL)12 chemokine gradient in the front region. Furthermore, senescent cells increase the survival of cancer cells via CXCL12/CXCR4 signalling. An orthotopic xenograft in vivo model also shows higher lymphatic vessels involvement in the group co-transplanted with senescent cells and cancer cells. These findings suggest that senescent cells are actively involved in the collective invasion and metastasis of PTC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
-
- Solinas G., Marchesi F., Garlanda C., Mantovani A. & Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 29, 243–248 (2010). - PubMed
-
- Cavallaro U. & Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 4, 118–132 (2004). - PubMed
-
- Friedl P., Locker J., Sahai E. & Segall J. E. Classifying collective cancer cell invasion. Nat. Cell Biol. 14, 777–783 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

