TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice
- PMID: 28489079
- PMCID: PMC5436240
- DOI: 10.1038/ncomms15292
TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice
Abstract
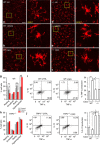
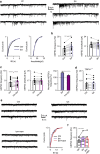
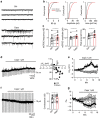
The capsaicin receptor TRPV1 has been widely characterized in the sensory system as a key component of pain and inflammation. A large amount of evidence shows that TRPV1 is also functional in the brain although its role is still debated. Here we report that TRPV1 is highly expressed in microglial cells rather than neurons of the anterior cingulate cortex and other brain areas. We found that stimulation of microglial TRPV1 controls cortical microglia activation per se and indirectly enhances glutamatergic transmission in neurons by promoting extracellular microglial microvesicles shedding. Conversely, in the cortex of mice suffering from neuropathic pain, TRPV1 is also present in neurons affecting their intrinsic electrical properties and synaptic strength. Altogether, these findings identify brain TRPV1 as potential detector of harmful stimuli and a key player of microglia to neuron communication.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Caterina M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). - PubMed
-
- Szallasi A. & Blumberg P. M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 51, 159–212 (1999). - PubMed
-
- Van Der Stelt M. & Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 271, 1827–1834 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

