N2 -to-NH3 Conversion by a triphos-Iron Catalyst and Enhanced Turnover under Photolysis
- PMID: 28489303
- PMCID: PMC5595421
- DOI: 10.1002/anie.201703244
N2 -to-NH3 Conversion by a triphos-Iron Catalyst and Enhanced Turnover under Photolysis
Abstract
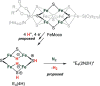
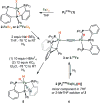
Bridging iron hydrides are proposed to form at the active site of MoFe-nitrogenase during catalytic dinitrogen reduction to ammonia and may be key in the binding and activation of N2 via reductive elimination of H2 . This possibility inspires the investigation of well-defined molecular iron hydrides as precursors for catalytic N2 -to-NH3 conversion. Herein, we describe the synthesis and characterization of new P2P'Ph Fe(N2 )(H)x systems that are active for catalytic N2 -to-NH3 conversion. Most interestingly, we show that the yields of ammonia can be significantly increased if the catalysis is performed in the presence of mercury lamp irradiation. Evidence is provided to suggest that photo-elimination of H2 is one means by which the enhanced activity may arise.
Keywords: ammonia; hydrides; iron complexes; nitrogen fixation; photolysis.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
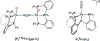
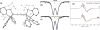

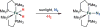
Similar articles
-
Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes.Chem Rev. 2020 Jun 24;120(12):5582-5636. doi: 10.1021/acs.chemrev.9b00638. Epub 2020 Apr 30. Chem Rev. 2020. PMID: 32352271 Free PMC article. Review.
-
Catalytic conversion of nitrogen to ammonia by an iron model complex.Nature. 2013 Sep 5;501(7465):84-7. doi: 10.1038/nature12435. Nature. 2013. PMID: 24005414 Free PMC article.
-
Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid.Science. 2016 Apr 22;352(6284):448-50. doi: 10.1126/science.aaf2091. Science. 2016. PMID: 27102481
-
Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia.Proc Natl Acad Sci U S A. 2016 May 17;113(20):5530-5. doi: 10.1073/pnas.1605512113. Epub 2016 May 2. Proc Natl Acad Sci U S A. 2016. PMID: 27140630 Free PMC article.
-
Insight into the Iron-Molybdenum Cofactor of Nitrogenase from Synthetic Iron Complexes with Sulfur, Carbon, and Hydride Ligands.J Am Chem Soc. 2016 Jun 15;138(23):7200-11. doi: 10.1021/jacs.6b00747. Epub 2016 Jun 3. J Am Chem Soc. 2016. PMID: 27171599 Free PMC article. Review.
Cited by
-
Dinitrogen Activation Mediated by the (P2PPh)Fe Complex: Electronic Structure, Dimerization Mechanism, and Magnetic Coupling.Inorg Chem. 2024 Jan 22;63(3):1633-1641. doi: 10.1021/acs.inorgchem.3c03813. Epub 2024 Jan 9. Inorg Chem. 2024. PMID: 38194669 Free PMC article.
-
Catalytic Nitrogen-to-Ammonia Conversion by Osmium and Ruthenium Complexes.J Am Chem Soc. 2017 Nov 15;139(45):16105-16108. doi: 10.1021/jacs.7b10204. Epub 2017 Nov 2. J Am Chem Soc. 2017. PMID: 29073760 Free PMC article.
-
Characterization of the Earliest Intermediate of Fe-N2 Protonation: CW and Pulse EPR Detection of an Fe-NNH Species and Its Evolution to Fe-NNH2.J Am Chem Soc. 2019 May 22;141(20):8116-8127. doi: 10.1021/jacs.8b12082. Epub 2019 May 14. J Am Chem Soc. 2019. PMID: 31046258 Free PMC article.
-
Fe-Mediated Nitrogen Fixation with a Metallocene Mediator: Exploring p Ka Effects and Demonstrating Electrocatalysis.J Am Chem Soc. 2018 May 16;140(19):6122-6129. doi: 10.1021/jacs.8b02335. Epub 2018 May 2. J Am Chem Soc. 2018. PMID: 29669205 Free PMC article.
-
Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes.Chem Rev. 2020 Jun 24;120(12):5582-5636. doi: 10.1021/acs.chemrev.9b00638. Epub 2020 Apr 30. Chem Rev. 2020. PMID: 32352271 Free PMC article. Review.
References
-
- Howard JB, Rees DC. Chem. Rev. 1996;96:2965–2982. - PubMed
-
- Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983–3012. - PubMed
-
- Yoo SJ, Angove HC, Papaefthymiou V, Burgess BK, Münck E. J. Am. Chem. Soc. 2000;122:4926–4936.
-
- Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696–1700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

