A Biphilic Phosphetane Catalyzes N-N Bond-Forming Cadogan Heterocyclization via PIII/PV═O Redox Cycling
- PMID: 28489354
- PMCID: PMC5776046
- DOI: 10.1021/jacs.7b03260
A Biphilic Phosphetane Catalyzes N-N Bond-Forming Cadogan Heterocyclization via PIII/PV═O Redox Cycling
Abstract
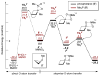
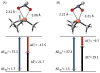
A small-ring phosphacycle, 1,2,2,3,4,4-hexamethylphosphetane, is found to catalyze deoxygenative N-N bond-forming Cadogan heterocyclization of o-nitrobenzaldimines, o-nitroazobenzenes, and related substrates in the presence of hydrosilane terminal reductant. The reaction provides a chemoselective catalytic synthesis of 2H-indazoles, 2H-benzotriazoles, and related fused heterocyclic systems with good functional group compatibility. On the basis of both stoichiometric and catalytic mechanistic experiments, the reaction is proposed to proceed via catalytic PIII/PV═O cycling, where DFT modeling suggests a turnover-limiting (3+1) cheletropic addition between the phosphetane catalyst and nitroarene substrate. Strain/distortion analysis of the (3+1) transition structure highlights the controlling role of frontier orbital effects underpinning the catalytic performance of the phosphetane.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Rowley AG. In: Organophosphorus Reagents in Organic Synthesis. Cadogan JIG, editor. Academic Press; London: 1979. p. 295.
-
- Maryanoff BE, Reitz AB. Chem Rev. 1989;89:863–927.
-
- Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem Rev. 2009;109:2551–2651. - PubMed
-
- Appel R. Angew Chem Int Ed Engl. 1975;14:801–811.
-
- Marsden SP. In: Sustainable Catalysis. Dunn PJ, Hii KK (Mimi), Krische MJ, Williams MT, editors. John Wiley & Sons, Inc; 2013. pp. 339–361.
- van Kalkeren HA, van Delft FL, Rutjes FPJT. ChemSusChem. 2013;6:1615–1624. - PubMed
- An J, Denton RM, Lambert TH, Nacsa ED. Org Biomol Chem. 2014;12:2993–3003. - PubMed
- Voituriez A, Saleh N. Tetrahedron Letters. 2016;57:4443–4451.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

