Hepatic Steatosis Accompanies Pulmonary Alveolar Proteinosis
- PMID: 28489415
- PMCID: PMC5650083
- DOI: 10.1165/rcmb.2016-0242OC
Hepatic Steatosis Accompanies Pulmonary Alveolar Proteinosis
Abstract
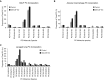
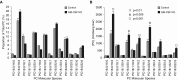
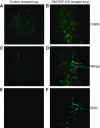
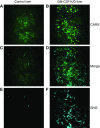
Maintenance of tissue-specific organ lipid compositions characterizes mammalian lipid homeostasis. The lungs and liver synthesize mixed phosphatidylcholine (PC) molecular species that are subsequently tailored for function. The lungs progressively enrich disaturated PC directed to lamellar body surfactant stores before secretion. The liver accumulates polyunsaturated PC directed to very-low-density lipoprotein assembly and secretion, or to triglyceride stores. In each tissue, selective PC species enrichment mechanisms lie at the heart of effective homeostasis. We tested for potential coordination between these spatially separated but possibly complementary phenomena under a major derangement of lung PC metabolism, pulmonary alveolar proteinosis (PAP), which overwhelms homeostasis and leads to excessive surfactant accumulation. Using static and dynamic lipidomics techniques, we compared (1) tissue PC compositions and contents, and (2) in lungs, the absolute rates of synthesis in both control mice and the granulocyte-macrophage colony-stimulating factor knockout model of PAP. Significant disaturated PC accumulation in bronchoalveolar lavage fluid, alveolar macrophage, and lavaged lung tissue occurred alongside increased PC synthesis, consistent with reported defects in alveolar macrophage surfactant turnover. However, microscopy using oil red O staining, coherent anti-Stokes Raman scattering, second harmonic generation, and transmission electron microscopy also revealed neutral-lipid droplet accumulations in alveolar lipofibroblasts of granular macrophage colony-stimulating factor knockout animals, suggesting that lipid homeostasis deficits extend beyond alveolar macrophages. PAP plasma PC composition was significantly polyunsaturated fatty acid enriched, but the content was unchanged and hepatic polyunsaturated fatty acid-enriched PC content increased by 50% with an accompanying micro/macrovesicular steatosis and a fibrotic damage pattern consistent with nonalcoholic fatty liver disease. These data suggest a hepatopulmonary axis of PC metabolism coordination, with wider implications for understanding and managing lipid pathologies in which compromise of one organ has unexpected consequences for another.
Keywords: fibrotic damage; hepatic steatosis; lipidomics; lipotoxicity; pulmonary alveolar proteinosis.
Figures



References
-
- Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. J Biol Chem. 1996;271:20219–20222. - PubMed
-
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. - PubMed
-
- Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50–56. - PubMed
-
- Haffar T, Bérubé-Simard F, Bousette N. Impaired fatty acid oxidation as a cause for lipotoxicity in cardiomyocytes. Biochem Biophys Res Commun. 2015;468:73–78. - PubMed
-
- Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, Soulage CO. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013;95:1971–1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

