Ephrin-B2/Fc promotes proliferation and migration, and suppresses apoptosis in human umbilical vein endothelial cells
- PMID: 28489586
- PMCID: PMC5522204
- DOI: 10.18632/oncotarget.17298
Ephrin-B2/Fc promotes proliferation and migration, and suppresses apoptosis in human umbilical vein endothelial cells
Abstract
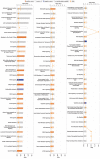
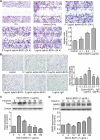
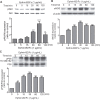
Tumor growth and metastasis are angiogenesis dependent. Angiogenic growth involves endothelial cell proliferation, migration, and invasion. Ephrin-B2 is a ligand for Eph receptor tyrosine kinases and is an important mediator in vascular endothelial growth factor-mediated angiogenesis. However, research offer controversial information regarding effects of ephrin-B2 on vascular endothelial cells. In this paper, proteome analyses showed that ephrin-B2/Fc significantly activates multiple signaling pathways related to cell proliferation, survival, and migration and suppresses apoptosis and cell death. Cytological experiments further confirm that ephrin-B2/Fc stimulates endothelial cell proliferation, triggers dose-dependent migration, and suppresses cell apoptosis. Results demonstrate that soluble dose-dependent ephrinB2 can promote proliferation and migration and inhibit apoptosis of human umbilical vein endothelial cells. These results also suggest that ephrinB2 prevents ischemic disease and can potentially be a new therapeutic target for treating angiogenesis-related diseases and tumors.
Keywords: ephrin-B2; human umbilical vein endothelial cell; migration; proliferation; proteomic analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Eph-B2/ephrin-B2 interaction plays a major role in the adhesion and proliferation of Waldenstrom's macroglobulinemia.Clin Cancer Res. 2012 Jan 1;18(1):91-104. doi: 10.1158/1078-0432.CCR-11-0111. Epub 2011 Oct 18. Clin Cancer Res. 2012. PMID: 22010211
-
Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro.J Vasc Surg. 2016 Mar;63(3):795-804. doi: 10.1016/j.jvs.2014.09.036. Epub 2014 Oct 24. J Vasc Surg. 2016. PMID: 25446283 Free PMC article.
-
Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells.J Cell Sci. 2003 Jun 15;116(Pt 12):2461-70. doi: 10.1242/jcs.00426. Epub 2003 May 6. J Cell Sci. 2003. PMID: 12734395
-
Regulation of signaling interactions and receptor endocytosis in growing blood vessels.Cell Adh Migr. 2014;8(4):366-77. doi: 10.4161/19336918.2014.970010. Cell Adh Migr. 2014. PMID: 25482636 Free PMC article. Review.
-
Essential roles of EphrinB2 in mammalian heart: from development to diseases.Cell Commun Signal. 2019 Mar 25;17(1):29. doi: 10.1186/s12964-019-0337-3. Cell Commun Signal. 2019. PMID: 30909943 Free PMC article. Review.
Cited by
-
Endothelial Notch signaling directly regulates the small GTPase RND1 to facilitate Notch suppression of endothelial migration.Sci Rep. 2022 Jan 31;12(1):1655. doi: 10.1038/s41598-022-05666-1. Sci Rep. 2022. PMID: 35102202 Free PMC article.
-
EphrinB2 overexpression enhances osteogenic differentiation of dental pulp stem cells partially through ephrinB2-mediated reverse signaling.Stem Cell Res Ther. 2020 Jan 29;11(1):40. doi: 10.1186/s13287-019-1540-2. Stem Cell Res Ther. 2020. PMID: 31996240 Free PMC article.
-
Vascular Injury in the Zebrafish Tail Modulates Blood Flow and Peak Wall Shear Stress to Restore Embryonic Circular Network.Front Cardiovasc Med. 2022 Mar 18;9:841101. doi: 10.3389/fcvm.2022.841101. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35369301 Free PMC article.
-
Head-to-head comparison of relevant cell sources of small extracellular vesicles for cardiac repair: Superiority of embryonic stem cells.J Extracell Vesicles. 2024 May;13(5):e12445. doi: 10.1002/jev2.12445. J Extracell Vesicles. 2024. PMID: 38711334 Free PMC article.
-
Ephs and Ephrins in Adult Endothelial Biology.Int J Mol Sci. 2020 Aug 6;21(16):5623. doi: 10.3390/ijms21165623. Int J Mol Sci. 2020. PMID: 32781521 Free PMC article. Review.
References
-
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. - PubMed
-
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

