Molecular characterization of circulating tumor cells from patients with metastatic breast cancer reflects evolutionary changes in gene expression under the pressure of systemic therapy
- PMID: 28489591
- PMCID: PMC5542207
- DOI: 10.18632/oncotarget.17271
Molecular characterization of circulating tumor cells from patients with metastatic breast cancer reflects evolutionary changes in gene expression under the pressure of systemic therapy
Abstract
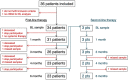
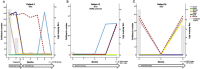
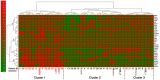
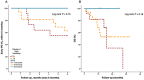
Resistance to systemic therapy is a major problem in metastatic breast cancer (MBC) that can be explained by initial tumor heterogeneity as well as by evolutionary changes during therapy and tumor progression. Circulating tumor cells (CTCs) detected in a liquid biopsy can be sampled and characterized repeatedly during therapy in order to monitor treatment response and disease progression.Our aim was to investigate how CTC derived gene expression of treatment predictive markers (ESR1/HER2) and other cancer associated markers changed in patient blood samples during six months of first-line systemic treatment for MBC. CTCs from 36 patients were enriched using CellSearch (Janssen Diagnostics) and AdnaTest (QIAGEN) before gene expression analysis was performed with a customized gene panel (TATAA Biocenter).Our results show that antibodies against HER2 and EGFR were valuable to isolate CTCs unidentified by CellSearch and possibly lacking EpCAM expression. Evaluation of patients with clinically different breast cancer subgroups demonstrated that gene expression of treatment predictive markers changed over time. This change was especially prominent for HER2 expression.In conclusion, we found that changed gene expression during first-line systemic therapy for MBC could be a possible explanation for treatment resistance. Characterization of CTCs at several time-points during therapy could be informative for treatment selection.
Keywords: Estrogen receptor (ER); circulating tumor cells; gene expression; human epidermal growth factor receptor 2 (HER2); metastatic breast cancer.
Conflict of interest statement
Vendula Novosadova is a shareholder in TATAA Biocenter. However, TATAA Biocenter had no role in the design of the study protocol, interpretation of data or any conclusions drawn from the study. The other authors have no conflicts of interest to declare.
Figures



References
-
- Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

