Oleate-induced PTX3 promotes head and neck squamous cell carcinoma metastasis through the up-regulation of vimentin
- PMID: 28489600
- PMCID: PMC5522334
- DOI: 10.18632/oncotarget.17326
Oleate-induced PTX3 promotes head and neck squamous cell carcinoma metastasis through the up-regulation of vimentin
Abstract
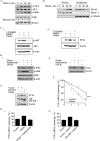
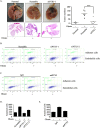
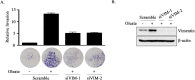
The association between metabolic diseases and the risk of developing cancer is emerging. However, the impact of long pentraxin-3 (PTX3) on dyslipidemia-associated tumor metastasis remains unknown. In this study, we found that oleate induced PTX3 expression and secretion through the activation of Akt/NF-κB pathway in head and neck squamous cell carcinomas (HNSCCs). The activation of NF-κB was essential for the oleate-induced stabilization of PTX3 mRNA. In addition, both the depletion of PTX3 and the inhibition of NF-κB significantly inhibited oleate-induced tumor cell migration and invasion. The enhancement of binding between tumor and endothelial cells was observed in oleate-treated cells but not in the depletion and neutralization of PTX3 with siPTX3 and anti-PTX3 antibodies, respectively. The levels of oleate-induced epithelial-mesenchymal transition (EMT) markers, such as vimentin and MMP-3, were significantly reduced in PTX3-depleted cells. Knocking down vimentin also repressed oleate-induced HNSCC invasion. Furthermore, the depletion of PTX3 blocked the oleate-primed metastatic seeding of tumor cells in the lungs. These results demonstrate that oleate enhances HNSCC metastasis through the PTX3/vimentin signaling axes. The inhibition of PTX3 could be a potential strategy for the treatment of dyslipidemia-mediated HNSCC metastasis.
Keywords: PTX3; metastasis; oleate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Oleic acid-induced ANGPTL4 enhances head and neck squamous cell carcinoma anoikis resistance and metastasis via up-regulation of fibronectin.Cancer Lett. 2017 Feb 1;386:110-122. doi: 10.1016/j.canlet.2016.11.012. Epub 2016 Nov 16. Cancer Lett. 2017. PMID: 27865799
-
PTX3 gene activation in EGF-induced head and neck cancer cell metastasis.Oncotarget. 2015 Apr 10;6(10):7741-57. doi: 10.18632/oncotarget.3482. Oncotarget. 2015. PMID: 25797258 Free PMC article.
-
p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma.Oncotarget. 2016 Jun 14;7(24):36539-36550. doi: 10.18632/oncotarget.9282. Oncotarget. 2016. PMID: 27174914 Free PMC article.
-
Epithelial-to-mesenchymal transition and cancer stem(-like) cells in head and neck squamous cell carcinoma.Cancer Lett. 2013 Sep 10;338(1):47-56. doi: 10.1016/j.canlet.2012.06.013. Epub 2012 Jul 4. Cancer Lett. 2013. PMID: 22771535 Review.
-
Genetic signature and profiling of head and neck cancer: where do we stand?Curr Opin Otolaryngol Head Neck Surg. 2017 Apr;25(2):154-158. doi: 10.1097/MOO.0000000000000348. Curr Opin Otolaryngol Head Neck Surg. 2017. PMID: 28141602 Review.
Cited by
-
A secretome profile indicative of oleate-induced proliferation of HepG2 hepatocellular carcinoma cells.Exp Mol Med. 2018 Aug 3;50(8):1-14. doi: 10.1038/s12276-018-0120-3. Exp Mol Med. 2018. PMID: 30076294 Free PMC article.
-
Role of long pentraxin PTX3 in cancer.Clin Exp Med. 2023 Dec;23(8):4401-4411. doi: 10.1007/s10238-023-01137-7. Epub 2023 Jul 12. Clin Exp Med. 2023. PMID: 37438568 Review.
-
Development of an Immunogenomic Landscape-Based Prognostic Index of Head and Neck Squamous Cell Carcinoma.Front Mol Biosci. 2020 Nov 24;7:586344. doi: 10.3389/fmolb.2020.586344. eCollection 2020. Front Mol Biosci. 2020. PMID: 33330624 Free PMC article.
-
PTX3 mediates the infiltration, migration, and inflammation-resolving-polarization of macrophages in glioblastoma.CNS Neurosci Ther. 2022 Nov;28(11):1748-1766. doi: 10.1111/cns.13913. Epub 2022 Jul 20. CNS Neurosci Ther. 2022. PMID: 35855654 Free PMC article.
-
Modulation of de Novo Lipogenesis Improves Response to Enzalutamide Treatment in Prostate Cancer.Cancers (Basel). 2020 Nov 11;12(11):3339. doi: 10.3390/cancers12113339. Cancers (Basel). 2020. PMID: 33187317 Free PMC article.
References
-
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. - PubMed
-
- Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–816. - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
-
- Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32:1766–1770. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

