Improved HIV-1 Viral Load Monitoring Capacity Using Pooled Testing With Marker-Assisted Deconvolution
- PMID: 28489730
- PMCID: PMC5503773
- DOI: 10.1097/QAI.0000000000001424
Improved HIV-1 Viral Load Monitoring Capacity Using Pooled Testing With Marker-Assisted Deconvolution
Abstract
Objective: Improve pooled viral load (VL) testing to increase HIV treatment monitoring capacity, particularly relevant for resource-limited settings.
Design: We developed marker-assisted mini-pooling with algorithm (mMPA), a new VL pooling deconvolution strategy that uses information from low-cost, routinely collected clinical markers to determine an efficient order of sequential individual VL testing and dictates when the sequential testing can be stopped.
Methods: We simulated the use of pooled testing to ascertain virological failure status on 918 participants from 3 studies conducted at the Academic Model Providing Access to Healthcare in Eldoret, Kenya, and estimated the number of assays needed when using mMPA and other pooling methods. We also evaluated the impact of practical factors, such as specific markers used, prevalence of virological failure, pool size, VL measurement error, and assay detection cutoffs on mMPA, other pooling methods, and single testing.
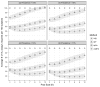
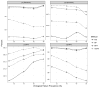
Results: Using CD4 count as a marker to assist deconvolution, mMPA significantly reduces the number of VL assays by 52% [confidence interval (CI): 48% to 57%], 40% (CI: 38% to 42%), and 19% (CI: 15% to 22%) compared with individual testing, simple mini-pooling, and mini-pooling with algorithm, respectively. mMPA has higher sensitivity and negative/positive predictive values than mini-pooling with algorithm, and comparable high specificity. Further improvement is achieved with additional clinical markers, such as age and time on therapy, with or without CD4 values. mMPA performance depends on prevalence of virological failure and pool size but is insensitive to VL measurement error and VL assay detection cutoffs.
Conclusions: mMPA can substantially increase the capacity of VL monitoring.
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Figures

References
-
- Anderson AM, Bartlett JA. Changing antiretroviral therapy in the setting of virologic relapse: Review of the current literature. Current HIV/AIDS Reports. 2006;3:79–85. - PubMed
-
- Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: Optional or necessary? Clinical Infectious Diseases. 2007;44(1):128–134. - PubMed
-
- Vekemans M, John L, Colebunders R. When to switch for antiretroviral treatment failure in resource-limited settings? AIDS. 2007;21:1205–1206. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2016. [Accessed on 9/12/2016]. ( http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf)
-
- WHO. [Accessed on 7/8/2016];Technical and operational considerations for implementing HIV viral load testing. 2016 ( http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

