Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure
- PMID: 28489753
- PMCID: PMC5428587
- DOI: 10.1097/MD.0000000000006769
Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure
Abstract
Background: This study aimed to determine the maximum tolerated dose (MTD), dose-limiting toxicity, and efficacy of second-line chemotherapy with FOLFIRINOX after gemcitabine (GEM)-based chemotherapy failure in metastatic pancreatic cancer (MPC).
Methods: We studied 18 histopathologically proven MPC patients. The schedule was 85 mg/m oxaliplatin, irinotecan, and 400 mg/m leucovorin, followed by 400 mg/m 5-fluorouracil (5-FU) as a bolus on day 1 and 2400 mg/m 5-FU as a 46-hour continuous infusion biweekly. The dose of irinotecan was defined as follows: level 0: 100 mg/m, level 1: 125 mg/m, level 2: 150 mg/m, and level 3: 180 mg/m. The doses of other drugs were fixed. The primary endpoint of phase II study was the response rate (RR).
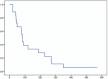
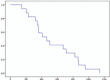
Results: We initially evaluated 6 patients in a phase I study. One patient developed neutropenia and 1 patient developed hyperglycemia and severe infection. Accordingly, level 1 was chosen as the MTD. According to a phase II study, the RR was 22.2% and the disease control rate was 61.1%. The progression-free survival and overall survival were 2.8 (range, 0.7-19.1) and 9.8 (2.4-19.8) months, respectively. The most common severe adverse event was neutropenia (66.7%). Febrile neutropenia occurred in 1 (5.6%) case.
Conclusion: The recommended dose was 85 mg/m oxaliplatin, 100 mg/m irinotecan, and 400 mg/m leucovorin, followed by 400 mg/m 5-FU as a bolus on day 1 and 2400 mg/m 5-FU as a 46-hour continuous infusion. These results indicate that second-line FOLFIRINOX is a marginally effective treatment for GEM-based chemotherapy failure cases.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures

References
-
- Rahib L, Smith B, Alzenberg R, et al. Projecting cancer incidence and deaths to 2030; the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605–17. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;5:2403–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

