Selectivity determinants of GPCR-G-protein binding
- PMID: 28489817
- PMCID: PMC5846738
- DOI: 10.1038/nature22070
Selectivity determinants of GPCR-G-protein binding
Abstract
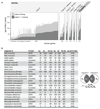
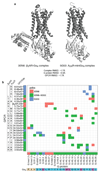
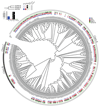
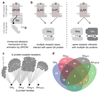
The selective coupling of G-protein-coupled receptors (GPCRs) to specific G proteins is critical to trigger the appropriate physiological response. However, the determinants of selective binding have remained elusive. Here we reveal the existence of a selectivity barcode (that is, patterns of amino acids) on each of the 16 human G proteins that is recognized by distinct regions on the approximately 800 human receptors. Although universally conserved positions in the barcode allow the receptors to bind and activate G proteins in a similar manner, different receptors recognize the unique positions of the G-protein barcode through distinct residues, like multiple keys (receptors) opening the same lock (G protein) using non-identical cuts. Considering the evolutionary history of GPCRs allows the identification of these selectivity-determining residues. These findings lay the foundation for understanding the molecular basis of coupling selectivity within individual receptors and G proteins.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







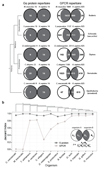

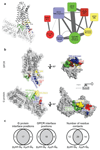


Comment in
-
Coding GPCR-G protein specificity.Cell Res. 2017 Oct;27(10):1193-1194. doi: 10.1038/cr.2017.92. Epub 2017 Jul 11. Cell Res. 2017. PMID: 28695889 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

