Whole-brain serial-section electron microscopy in larval zebrafish
- PMID: 28489821
- PMCID: PMC5594570
- DOI: 10.1038/nature22356
Whole-brain serial-section electron microscopy in larval zebrafish
Abstract
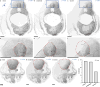
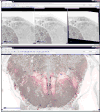
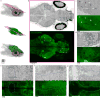
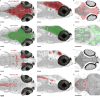
High-resolution serial-section electron microscopy (ssEM) makes it possible to investigate the dense meshwork of axons, dendrites, and synapses that form neuronal circuits. However, the imaging scale required to comprehensively reconstruct these structures is more than ten orders of magnitude smaller than the spatial extents occupied by networks of interconnected neurons, some of which span nearly the entire brain. Difficulties in generating and handling data for large volumes at nanoscale resolution have thus restricted vertebrate studies to fragments of circuits. These efforts were recently transformed by advances in computing, sample handling, and imaging techniques, but high-resolution examination of entire brains remains a challenge. Here, we present ssEM data for the complete brain of a larval zebrafish (Danio rerio) at 5.5 days post-fertilization. Our approach utilizes multiple rounds of targeted imaging at different scales to reduce acquisition time and data management requirements. The resulting dataset can be analysed to reconstruct neuronal processes, permitting us to survey all myelinated axons (the projectome). These reconstructions enable precise investigations of neuronal morphology, which reveal remarkable bilateral symmetry in myelinated reticulospinal and lateral line afferent axons. We further set the stage for whole-brain structure-function comparisons by co-registering functional reference atlases and in vivo two-photon fluorescence microscopy data from the same specimen. All obtained images and reconstructions are provided as an open-access resource.
Figures









References
-
- Briggman KL, Bock DD. Volume electron microscopy for neuronal circuit reconstruction. Curr. Opin. Neurobiol. 2012;22:154–161. - PubMed
-
- Lichtman JW, Denk W. The big and the small: challenges of imaging the brain's circuits. Science. 2011;334:618–623. - PubMed
-
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R Soc. Lond. B. 1986;314:1–340. - PubMed
-
- Jarrell TA, et al. The connectome of a decision-making neural network. Science. 2012;337:437–444. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

