Generational distribution of a Candida glabrata population: Resilient old cells prevail, while younger cells dominate in the vulnerable host
- PMID: 28489916
- PMCID: PMC5440053
- DOI: 10.1371/journal.ppat.1006355
Generational distribution of a Candida glabrata population: Resilient old cells prevail, while younger cells dominate in the vulnerable host
Abstract
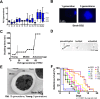
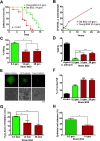
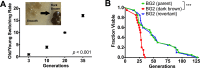
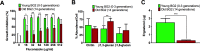
Similar to other yeasts, the human pathogen Candida glabrata ages when it undergoes asymmetric, finite cell divisions, which determines its replicative lifespan. We sought to investigate if and how aging changes resilience of C. glabrata populations in the host environment. Our data demonstrate that old C. glabrata are more resistant to hydrogen peroxide and neutrophil killing, whereas young cells adhere better to epithelial cell layers. Consequently, virulence of old compared to younger C. glabrata cells is enhanced in the Galleria mellonella infection model. Electron microscopy images of old C. glabrata cells indicate a marked increase in cell wall thickness. Comparison of transcriptomes of old and young C. glabrata cells reveals differential regulation of ergosterol and Hog pathway associated genes as well as adhesion proteins, and suggests that aging is accompanied by remodeling of the fungal cell wall. Biochemical analysis supports this conclusion as older cells exhibit a qualitatively different lipid composition, leading to the observed increased emergence of fluconazole resistance when grown in the presence of fluconazole selection pressure. Older C. glabrata cells accumulate during murine and human infection, which is statistically unlikely without very strong selection. Therefore, we tested the hypothesis that neutrophils constitute the predominant selection pressure in vivo. When we altered experimentally the selection pressure by antibody-mediated removal of neutrophils, we observed a significantly younger pathogen population in mice. Mathematical modeling confirmed that differential selection of older cells is sufficient to cause the observed demographic shift in the fungal population. Hence our data support the concept that pathogenesis is affected by the generational age distribution of the infecting C. glabrata population in a host. We conclude that replicative aging constitutes an emerging trait, which is selected by the host and may even play an unanticipated role in the transition from a commensal to a pathogen state.
Conflict of interest statement
Maurizio Del Poeta is the Chief Scientific Officer of MicroRid Technologies, Inc.
Figures
Similar articles
-
Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy.Virulence. 2017 Nov 17;8(8):1909-1917. doi: 10.1080/21505594.2017.1347744. Epub 2017 Aug 1. Virulence. 2017. PMID: 28658597 Free PMC article. No abstract available.
-
Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies.Mol Microbiol. 2006 Aug;61(3):704-22. doi: 10.1111/j.1365-2958.2006.05235.x. Epub 2006 Jun 27. Mol Microbiol. 2006. PMID: 16803598
-
Clumping Morphology Influences Virulence Uncoupled from Echinocandin Resistance in Candida glabrata.Microbiol Spectr. 2022 Feb 23;10(1):e0183721. doi: 10.1128/spectrum.01837-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107318 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
A yeast by any other name: Candida glabrata and its interaction with the host.Curr Opin Microbiol. 2005 Aug;8(4):378-84. doi: 10.1016/j.mib.2005.06.012. Curr Opin Microbiol. 2005. PMID: 15996895 Review.
Cited by
-
Fungi's Swiss Army Knife: Pleiotropic Effect of Melanin in Fungal Pathogenesis during Cattle Mycosis.J Fungi (Basel). 2023 Sep 15;9(9):929. doi: 10.3390/jof9090929. J Fungi (Basel). 2023. PMID: 37755037 Free PMC article.
-
High-Throughput Yeast Aging Analysis for Cryptococcus (HYAAC) microfluidic device streamlines aging studies in Cryptococcus neoformans.Commun Biol. 2019 Jul 10;2:256. doi: 10.1038/s42003-019-0504-5. eCollection 2019. Commun Biol. 2019. PMID: 31312725 Free PMC article.
-
The contribution of Saccharomyces cerevisiae replicative age to the variations in the levels of Trx2p, Pdr5p, Can1p and Idh isoforms.Sci Rep. 2017 Oct 16;7(1):13220. doi: 10.1038/s41598-017-13576-w. Sci Rep. 2017. PMID: 29038504 Free PMC article.
-
Cell Wall-Associated Virulence Factors Contribute to Increased Resilience of Old Cryptococcus neoformans Cells.Front Microbiol. 2019 Nov 7;10:2513. doi: 10.3389/fmicb.2019.02513. eCollection 2019. Front Microbiol. 2019. PMID: 31787940 Free PMC article.
-
Combined Antifungal Resistance and Biofilm Tolerance: the Global Threat of Candida auris.mSphere. 2019 Jul 31;4(4):e00458-19. doi: 10.1128/mSphere.00458-19. mSphere. 2019. PMID: 31366705 Free PMC article. Review.
References
-
- Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagnostic microbiology and infectious disease. 2012;73(1):45–8. Epub 2012/05/15. doi: 10.1016/j.diagmicrobio.2012.02.001 - DOI - PubMed
-
- Wang E, Farmakiotis D, Yang D, McCue DA, Kantarjian HM, Kontoyiannis DP, et al. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother. 2015;70(8):2362–8. doi: 10.1093/jac/dkv087 - DOI - PMC - PubMed
-
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS microbiology reviews. 2012;36(2):288–305. Epub 2011/05/17. doi: 10.1111/j.1574-6976.2011.00278.x - DOI - PubMed
-
- Cuellar-Cruz M, Briones-Martin-del-Campo M, Canas-Villamar I, Montalvo-Arredondo J, Riego-Ruiz L, Castano I, et al. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryotic cell. 2008;7(5):814–25. Epub 2008/04/01. PubMed Central PMCID: PMC2394966. doi: 10.1128/EC.00011-08 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

