Opposing Effects of Valproic Acid Treatment Mediated by Histone Deacetylase Inhibitor Activity in Four Transgenic X. laevis Models of Retinitis Pigmentosa
- PMID: 28490005
- PMCID: PMC6597025
- DOI: 10.1523/JNEUROSCI.1647-16.2016
Opposing Effects of Valproic Acid Treatment Mediated by Histone Deacetylase Inhibitor Activity in Four Transgenic X. laevis Models of Retinitis Pigmentosa
Abstract
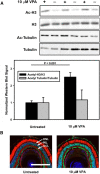
Retinitis pigmentosa (RP) is an inherited retinal degeneration (RD) that leads to blindness for which no treatment is available. RP is frequently caused by mutations in Rhodopsin; in some animal models, RD is exacerbated by light. Valproic acid (VPA) is a proposed treatment for RP and other neurodegenerative disorders, with a phase II trial for RP under way. However, the therapeutic mechanism is unclear, with minimal research supporting its use in RP. We investigated the effects of VPA on Xenopus laevis models of RP expressing human P23H, T17M, T4K, and Q344ter rhodopsins, which are associated with RP in humans. VPA ameliorated RD associated with P23H rhodopsin and promoted clearing of mutant rhodopsin from photoreceptors. The effect was equal to that of dark rearing, with no additive effect observed. Rescue of visual function was confirmed by electroretinography. In contrast, VPA exacerbated RD caused by T17M rhodopsin in light, but had no effect in darkness. Effects in T4K and Q344ter rhodopsin models were also negative. These effects of VPA were paralleled by treatment with three additional histone deacetylase (HDAC) inhibitors, but not other antipsychotics, chemical chaperones, or VPA structural analogues. In WT retinas, VPA treatment increased histone H3 acetylation. In addition, electron microscopy showed increased autophagosomes in rod inner segments with HDAC inhibitor (HDACi) treatment, potentially linking the therapeutic effects in P23H rhodopsin animals and negative effects in other models with autophagy. Our results suggest that the success or failure of VPA treatment is dependent on genotype and that HDACi treatment is contraindicated for some RP cases.SIGNIFICANCE STATEMENT Retinitis pigmentosa (RP) is an inherited, degenerative retinal disease that leads to blindness for which no therapy is available. We determined that valproic acid (VPA), currently undergoing a phase II trial for RP, has both beneficial and detrimental effects in animal models of RP depending on the underlying disease mechanism and that both effects are due to histone deacetylase (HDAC) inhibition possibly linked to autophagy regulation. Off-label use of VPA and other HDAC inhibitors for the treatment of RP should be limited to the research setting until this effect is understood and can be predicted. Our study suggests that, unless genotype is accounted for, clinical trials for RP treatments may give negative results due to multiple disease mechanisms with differential responses to therapeutic interventions.
Keywords: HDAC inhibitor; autophagy; retinal degeneration; retinitis pigmentosa; rhodopsin; valproic acid.
Copyright © 2017 the authors 0270-6474/17/371039-16$15.00/0.
Figures
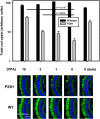

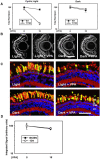
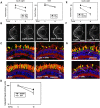

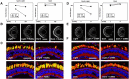


Comment in
-
Valproic Acid for a Treatment of Retinitis Pigmentosa: Reasons for Optimism and Caution.J Neurosci. 2017 May 24;37(21):5215-5217. doi: 10.1523/JNEUROSCI.0774-17.2017. J Neurosci. 2017. PMID: 28539347 Free PMC article. No abstract available.
Similar articles
-
Photoactivation-induced instability of rhodopsin mutants T4K and T17M in rod outer segments underlies retinal degeneration in X. laevis transgenic models of retinitis pigmentosa.J Neurosci. 2014 Oct 1;34(40):13336-48. doi: 10.1523/JNEUROSCI.1655-14.2014. J Neurosci. 2014. PMID: 25274813 Free PMC article.
-
Autophagy Induction by HDAC Inhibitors Is Unlikely to be the Mechanism of Efficacy in Prevention of Retinal Degeneration Caused by P23H Rhodopsin.Adv Exp Med Biol. 2019;1185:401-405. doi: 10.1007/978-3-030-27378-1_66. Adv Exp Med Biol. 2019. PMID: 31884645
-
Dark rearing rescues P23H rhodopsin-induced retinal degeneration in a transgenic Xenopus laevis model of retinitis pigmentosa: a chromophore-dependent mechanism characterized by production of N-terminally truncated mutant rhodopsin.J Neurosci. 2007 Aug 22;27(34):9043-53. doi: 10.1523/JNEUROSCI.2245-07.2007. J Neurosci. 2007. PMID: 17715341 Free PMC article.
-
The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy.Prog Retin Eye Res. 2018 Jan;62:1-23. doi: 10.1016/j.preteyeres.2017.10.002. Epub 2017 Oct 16. Prog Retin Eye Res. 2018. PMID: 29042326 Free PMC article. Review.
-
Targeting the Proteostasis Network in Rhodopsin Retinitis Pigmentosa.Adv Exp Med Biol. 2016;854:479-84. doi: 10.1007/978-3-319-17121-0_64. Adv Exp Med Biol. 2016. PMID: 26427449 Free PMC article. Review.
Cited by
-
Subcellular localization of mutant P23H rhodopsin in an RFP fusion knock-in mouse model of retinitis pigmentosa.Dis Model Mech. 2022 May 1;15(5):dmm049336. doi: 10.1242/dmm.049336. Epub 2022 May 6. Dis Model Mech. 2022. PMID: 35275162 Free PMC article.
-
Balancing the Photoreceptor Proteome: Proteostasis Network Therapeutics for Inherited Retinal Disease.Genes (Basel). 2019 Jul 24;10(8):557. doi: 10.3390/genes10080557. Genes (Basel). 2019. PMID: 31344897 Free PMC article. Review.
-
Exploring Histone Modifications in Inherited Retinal Disorders.Adv Exp Med Biol. 2025;1468:189-193. doi: 10.1007/978-3-031-76550-6_31. Adv Exp Med Biol. 2025. PMID: 39930194 Review.
-
The role of epigenetic changes in the pathology and treatment of inherited retinal diseases.Front Cell Dev Biol. 2023 Aug 4;11:1224078. doi: 10.3389/fcell.2023.1224078. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37601102 Free PMC article. Review.
-
Autophagy in Xenopus laevis rod photoreceptors is independently regulated by phototransduction and misfolded RHOP23H.Autophagy. 2019 Nov;15(11):1970-1989. doi: 10.1080/15548627.2019.1596487. Epub 2019 Apr 12. Autophagy. 2019. PMID: 30975014 Free PMC article.
References
-
- Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. - PubMed
-
- Bogéa TH, Wen RH, Moritz OL. Light induces ultrastructural changes in rod outer and inner segments, including autophagy, in a transgenic Xenopus laevis P23H rhodopsin model of retinitis pigmentosa autophagy in P23H light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2015;56:7947–7955. doi: 10.1167/iovs.15-16799. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
