Rapid Homogeneous Immunoassay to Quantify Gemcitabine in Plasma for Therapeutic Drug Monitoring
- PMID: 28490046
- PMCID: PMC5460533
- DOI: 10.1097/FTD.0000000000000402
Rapid Homogeneous Immunoassay to Quantify Gemcitabine in Plasma for Therapeutic Drug Monitoring
Abstract
Background: Gemcitabine (2',2'-difluoro-2'-deoxycytidine) is a nucleoside analog used as a single agent and in combination regimens for the treatment of a variety of solid tumors. Several studies have shown a relationship between gemcitabine peak plasma concentration (Cmax) and hematological toxicity. An immunoassay for gemcitabine in plasma was developed and validated to facilitate therapeutic drug monitoring (TDM) by providing an economical, robust method for automated chemistry analyzers.
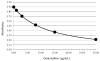
Methods: A monoclonal antibody was coated on nanoparticles to develop a homogenous agglutination inhibition assay. To prevent ex vivo degradation of gemcitabine in blood, tetrahydrouridine was used as a sample stabilizer. Validation was conducted for precision, recovery, cross-reactivity, and linearity on a Beckman Coulter AU480. Verification was performed on an AU5800 in a hospital laboratory. A method comparison was performed with (LC-MS/MS) liquid chromatography tandem mass spectrometry using clinical samples. Selectivity was demonstrated by testing cross-reactivity of the major metabolite, 2',2'-difluorodeoxyuridine.
Results: Coefficients of variation for repeatability and within-laboratory precision were <8%. The deviation between measured and assigned values was <3%. Linear range was from 0.40 to 33.02 μ/mL (1.5-125.5 μM). Correlation with validated LC-MS/MS methods was R = 0.977. The assay was specific for gemcitabine: there was no cross-reactivity to 2',2'-difluorodeoxyuridine, chemotherapeutics, concomitant, or common medications tested. Tetrahydrouridine was packaged in single-use syringes. Gemcitabine stability in whole blood was extended to 8 hours (at room temperature) and in plasma to 8 days (2-8°C).
Conclusions: The assay demonstrated the selectivity, test range, precision, and linearity to perform reliable measurements of gemcitabine in plasma. The addition of stabilizer improved the sample handling. Using general clinical chemistry analyzers, gemcitabine could be measured for TDM.
Figures



Similar articles
-
An automated nanoparticle-based homogeneous immunoassay for determining docetaxel concentrations in plasma.Ther Drug Monit. 2013 Dec;35(6):803-8. doi: 10.1097/FTD.0b013e31829617ea. Ther Drug Monit. 2013. PMID: 24263639
-
Validation of a hydrophilic interaction ultra-performance liquid chromatography-tandem mass spectrometry method for the determination of gemcitabine in human plasma with tetrahydrouridine.Biomed Chromatogr. 2015 Sep;29(9):1343-9. doi: 10.1002/bmc.3429. Epub 2015 Feb 2. Biomed Chromatogr. 2015. PMID: 25641274
-
Simultaneous determination of gemcitabine prodrug, gemcitabine and its major metabolite 2', 2'-difluorodeoxyuridine in rat plasma by UFLC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 May 1;1084:4-13. doi: 10.1016/j.jchromb.2018.03.025. Epub 2018 Mar 14. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 29558739
-
Evaluation of a Nanoparticle-Based Busulfan Immunoassay for Rapid Analysis on Routine Clinical Analyzers.Ther Drug Monit. 2021 Dec 1;43(6):766-771. doi: 10.1097/FTD.0000000000000883. Ther Drug Monit. 2021. PMID: 33814542 Free PMC article.
-
Determination of gemcitabine and its metabolite in extracellular fluid of rat brain tumor by ultra performance liquid chromatography-tandem mass spectrometry using microdialysis sampling after intralesional chemotherapy.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Mar 1;919-920:10-9. doi: 10.1016/j.jchromb.2012.12.027. Epub 2013 Jan 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 23396113
Cited by
-
Scaffold-based three-dimensional cell model of pancreatic cancer is more suitable than scaffold-free three-dimensional cell model of pancreatic cancer for drug discovery.Cytotechnology. 2022 Dec;74(6):657-667. doi: 10.1007/s10616-022-00553-z. Epub 2022 Oct 20. Cytotechnology. 2022. PMID: 36389286 Free PMC article.
-
Development of a UPLC-MS/MS method for simultaneous therapeutic drug monitoring of anti-hepatocellular carcinoma drugs and analgesics in human plasma.Front Pharmacol. 2023 May 31;14:1136735. doi: 10.3389/fphar.2023.1136735. eCollection 2023. Front Pharmacol. 2023. PMID: 37324468 Free PMC article.
-
Phase I study of veliparib in combination with gemcitabine.Cancer Chemother Pharmacol. 2017 Sep;80(3):631-643. doi: 10.1007/s00280-017-3409-3. Epub 2017 Aug 2. Cancer Chemother Pharmacol. 2017. PMID: 28770300 Free PMC article. Clinical Trial.
-
Rapid Enzymatic Assay for Antiretroviral Drug Monitoring Using CRISPR-Cas12a-Enabled Readout.ACS Synth Biol. 2025 Feb 21;14(2):510-519. doi: 10.1021/acssynbio.4c00674. Epub 2025 Feb 11. ACS Synth Biol. 2025. PMID: 39933068 Free PMC article.
-
Opportunities for Precision Dosing of Cytotoxic Drugs in Non-Small Cell Lung Cancer: Bridging the Gap in Precision Medicine.Clin Pharmacokinet. 2025 Apr;64(4):511-531. doi: 10.1007/s40262-025-01492-6. Epub 2025 Mar 5. Clin Pharmacokinet. 2025. PMID: 40045151 Free PMC article. Review.
References
-
- Baker SD, Verweij J, Rowinsky EK, et al. A Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst. 2002;94:1883–1888. - PubMed
-
- Felici A, Verweij J, Sparreboom A. Dosing strategies for anticancer drugs: the good, the bad and body-surface area. Eur J Cancer. 2002;38:1677–1684. - PubMed
-
- Peters GJ. Novel developments in the use of antimetabolites. Nucleosides Nucleotides Nucleic Acids. 2014;33:358–374. - PubMed
-
- Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

