Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka
- PMID: 28490339
- PMCID: PMC5424329
- DOI: 10.1186/s13071-017-2174-9
Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka
Abstract
Background: Wolbachia are a group of maternally inherited intracellular bacteria known to be widespread among arthropods. Infections with Wolbachia cause declines of host populations, and also induce host resistance to a wide range of pathogens. Over the past few decades, researchers were curious to use Wolbachia as a biological tool to control mosquito vectors. During the present study, assessment of the prevalence of Wolbachia infections among wild mosquito populations in Sri Lanka where mosquito-borne diseases are a major health concern, was carried out for the first time. DNA was extracted from the abdomens of mosquitoes, collected from seven provinces, and screened for the presence of Wolbachia by PCR using wsp and groE primers. Group-specific and strain-specific primers were used to classify Wolbachia into the supergroups A and B, and into the strains Mel, AlbA and Pip.
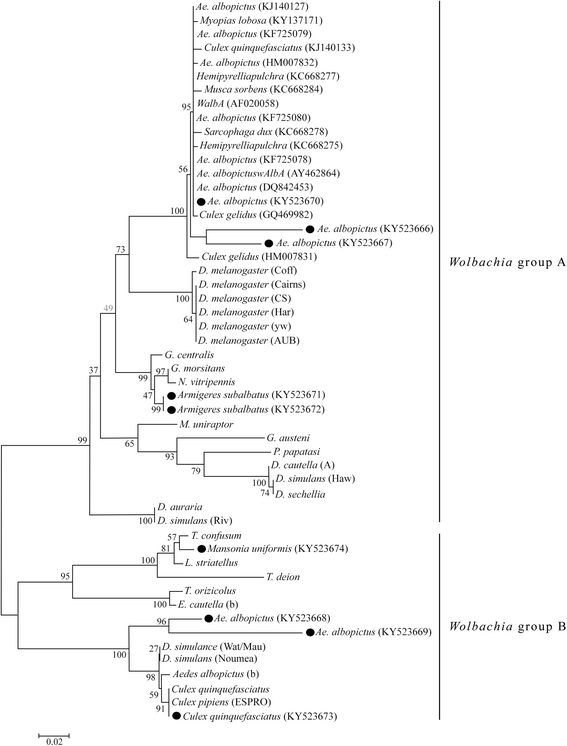
Results: A total of 330 individual mosquitoes belonging to 22 species and 7 genera were screened. Eighty-seven mosquitoes (26.36%) belonging to four species (i.e. Aedes albopictus, Culex quinquefasciatus, Armigeres subalbatus and Mansonia uniformis) were positive for Wolbachia infections. Primary vector of the dengue fever, Ae. aegypti was negative for Wolbachia infections while the secondary vector, Ae. albopictus, showed a very high infection rate. The filarial vector C. quinquefasciatus had a relatively high rate of infection. Japanese encephalitis vectors C. gelidus and C. triteaneorynchus, and the Anopheles vectors of malaria were negative for Wolbachia infections. Nine sequences of Wolbachia-positive PCR products were deposited in the GenBank and compared with other available data. Aedes albopictus was infected with both Wolbachia strains A (AlbA) and B (Pip) supergroups. Phylogenetic analysis of the wsp sequences showed two major branches confirming identities obtained from the PCR screening with strain-specific primers.
Conclusion: Wolbachia infections were found only among four mosquito species in Sri Lanka: Aedes albopictus, Culex quinquefasciatus, Armigeres subalbatus and Mansonia uniformis. Sequence data showed high haplotype diversity among the Wolbachia strains.
Keywords: Biological control; Mosquito control; Phylogeny; Sri Lanka; Wolbachia strains; Wsp.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

