Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury
- PMID: 28490444
- PMCID: PMC5496040
- DOI: 10.1194/jlr.M076745
Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury
Abstract
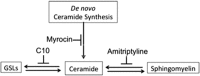
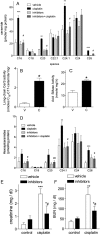
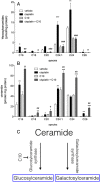
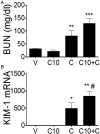
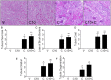
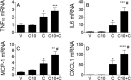
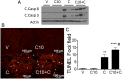
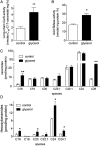
Acute kidney injury (AKI), resulting from chemotherapeutic agents such as cisplatin, remains an obstacle in the treatment of cancer. Cisplatin-induced AKI involves apoptotic and necrotic cell death, pathways regulated by sphingolipids such as ceramide and glucosylceramide. Results from this study indicate that C57BL/6J mice treated with cisplatin had increased ceramide and hexosylceramide levels in the renal cortex 72 h following cisplatin treatment. Pretreatment of mice with inhibitors of acid sphingomyelinase and de novo ceramide synthesis (amitriptyline and myriocin, respectively) prevented accumulation of ceramides and hexosylceramide in the renal cortex and protected from cisplatin-induced AKI. To determine the role of ceramide metabolism to hexosylceramides in kidney injury, we treated mice with a potent and highly specific inhibitor of glucosylceramide synthase, the enzyme responsible for catalyzing the glycosylation of ceramides to form glucosylceramides. Inhibition of glucosylceramide synthase attenuated the accumulation of the hexosylceramides and exacerbated ceramide accumulation in the renal cortex following treatment of mice with cisplatin. Increasing ceramides and decreasing glucosylceramides in the renal cortex sensitized mice to cisplatin-induced AKI according to markers of kidney function, kidney injury, inflammation, cell stress, and apoptosis. Under conditions of high ceramide generation, data suggest that metabolism of ceramides to glucosylceramides buffers kidney ceramides and helps attenuate kidney injury.-Dupre, T. V., M. A. Doll, P. P. Shah, C. N. Sharp, D. Siow, J. Megyesi, J. Shayman, A. Bielawska, J. Bielawski, L. J. Beverly, M. Hernandez-Corbacho, C. J. Clarke, A. J. Snider, R. G. Schnellmann, L. M. Obeid, Y. A. Hannun, and L. J. Siskind. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res 2017. 58: 1439-1452.
Keywords: apoptosis; ceramide; inflammation; sphingolipids.
Figures


References
-
- Bellomo R., Kellum J. A., and Ronco C.. 2012. Acute kidney injury. Lancet. 380: 756–766. - PubMed
-
- Liangos O., Wald R., O’Bell J. W., Price L., Pereira B. J., and Jaber B. L.. 2006. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin. J. Am. Soc. Nephrol. 1: 43–51. - PubMed
-
- Singri N., Ahya S. N., and Levin M. L.. 2003. Acute renal failure. JAMA. 289: 747–751. - PubMed
-
- Pabla N., and Dong Z.. 2008. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73: 994–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

