Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice
- PMID: 28490451
- PMCID: PMC5495920
- DOI: 10.1152/ajpregu.00511.2016
Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice
Abstract
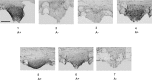
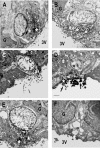
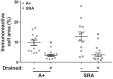
Direct intracerebroventricular injection of angiotensin II (ANG II) causes increases in blood pressure and salt and water intake, presumably mimicking an effect mediated by an endogenous mechanism. The subfornical organ (SFO) is a potential source of cerebrospinal fluid (CSF), ANG I, and ANG II, and thus we hypothesized that the SFO has a secretory function. Endogenous levels of angiotensinogen (AGT) and renin are very low in the brain. We therefore examined the immunohistochemical localization of angiotensin peptides and AGT in the SFO, and AGT in the CSF in two transgenic models that overexpress either human AGT (A+ mice), or both human AGT (hAGT) and human renin (SRA mice) in the brain. Measurements were made at baseline and following volumetric depletion of CSF. Ultrastructural analysis with immunoelectron microscopy revealed that superficially located ANG I/ANG II and AGT immunoreactive cells in the SFO were vacuolated and opened directly into the ventricle. Withdrawal of CSF produced an increase in AGT in the CSF that was accompanied by a large decline in AGT immunoreactivity within SFO cells. Our data provide support for the hypothesis that the SFO is a secretory organ that releases AGT and possibly ANG I/ANG II into the ventricle at least under conditions when genes that control the renin-angiotensin system are overexpressed in mice.
Keywords: angiotensinogen; cerebrospinal fluid; secretion; subfornical organ; transgenic mice.
Copyright © 2017 the American Physiological Society.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

