Vaccines to Accelerate Malaria Elimination and Eventual Eradication
- PMID: 28490535
- PMCID: PMC5580511
- DOI: 10.1101/cshperspect.a025627
Vaccines to Accelerate Malaria Elimination and Eventual Eradication
Abstract
Remarkable progress has been made in coordinated malaria control efforts with substantial reductions in malaria-associated deaths and morbidity achieved through mass administration of drugs and vector control measures including distribution of long-lasting insecticide-impregnated bednets and indoor residual spraying. However, emerging resistance poses a significant threat to the sustainability of these interventions. In this light, the malaria research community has been charged with the development of a highly efficacious vaccine to complement existing malaria elimination measures. As the past 40 years of investment in this goal attests, this is no small feat. The malaria parasite is a highly complex organism, exquisitely adapted for survival under hostile conditions within human and mosquito hosts. Here we review current vaccine strategies to accelerate elimination and the potential for novel and innovative approaches to vaccine design through a better understanding of the host-parasite interaction.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
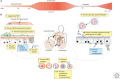
References
-
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, et al. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365: 1863–1875. - PubMed
-
- Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH. 2011. SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol Microbiol 79: 929–939. - PubMed
-
- Amino R, Thiberge S, Shorte S, Frischknecht F, Menard R. 2006. Quantitative imaging of Plasmodium sporozoites in the mammalian host. C R Biol 329: 858–862. - PubMed
-
- Arumugam TU, Ito D, Takashima E, Tachibana M, Ishino T, Torii M, Tsuboi T. 2014. Application of wheat germ cell-free protein expression system for novel malaria vaccine candidate discovery. Expert Rev Vaccines 13: 75–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
