Molecular Regulation of Exercise-Induced Muscle Fiber Hypertrophy
- PMID: 28490543
- PMCID: PMC5983156
- DOI: 10.1101/cshperspect.a029751
Molecular Regulation of Exercise-Induced Muscle Fiber Hypertrophy
Abstract
Skeletal muscle hypertrophy is a widely sought exercise adaptation to counteract the muscle atrophy of aging and disease, or to improve athletic performance. While this desired muscle enlargement is a well-known adaptation to resistance exercise training (RT), the mechanistic underpinnings are not fully understood. The purpose of this review is thus to provide the reader with a summary of recent advances in molecular mechanisms-based on the most current literature-that are thought to promote RT-induced muscle hypertrophy. We have therefore focused this discussion on the following areas of fertile investigation: ribosomal function and biogenesis, muscle stem (satellite) cell activity, transcriptional regulation, mechanotransduction, and myokine signaling.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
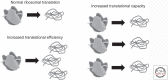
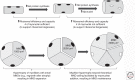

References
-
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, et al. 2005. Dystrophin glycoprotein complex dysfunction: A regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8: 421–432. - PubMed
-
- Adams GR, Bamman MM. 2012. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol 2: 2829–2870. - PubMed
-
- Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. 2007. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
