Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis
- PMID: 28490579
- PMCID: PMC5475225
- DOI: 10.1128/CMR.00013-17
Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis
Abstract
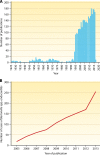
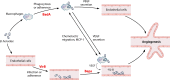
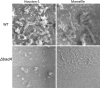
Since the reclassification of the genus Bartonella in 1993, the number of species has grown from 1 to 45 currently designated members. Likewise, the association of different Bartonella species with human disease continues to grow, as does the range of clinical presentations associated with these bacteria. Among these, blood-culture-negative endocarditis stands out as a common, often undiagnosed, clinical presentation of infection with several different Bartonella species. The limitations of laboratory tests resulting in this underdiagnosis of Bartonella endocarditis are discussed. The varied clinical picture of Bartonella infection and a review of clinical aspects of endocarditis caused by Bartonella are presented. We also summarize the current knowledge of the molecular basis of Bartonella pathogenesis, focusing on surface adhesins in the two Bartonella species that most commonly cause endocarditis, B. henselae and B. quintana. We discuss evidence that surface adhesins are important factors for autoaggregation and biofilm formation by Bartonella species. Finally, we propose that biofilm formation is a critical step in the formation of vegetative masses during Bartonella-mediated endocarditis and represents a potential reservoir for persistence by these bacteria.
Keywords: Bartonella; biofilm; blood-culture-negative endocarditis; emerging infections; trimeric autotransporter adhesins.
Copyright © 2017 American Society for Microbiology.
Figures






References
-
- Schulte Fischedick FB, Stuckey MJ, Aguilar-Setien A, Moreno-Sandoval H, Galvez-Romero G, Salas-Rojas M, Arechiga-Ceballos N, Overgaauw PA, Kasten RW, Chomel BB. 2016. Identification of Bartonella species isolated from rodents from Yucatan, Mexico, and isolation of Bartonella vinsonii subsp. yucatanensis subsp nov. Vector Borne Zoonotic Dis 16:636–642. doi: 10.1089/vbz.2016.1981. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

