Unexpected Efficacy of a Novel Sodium Channel Modulator in Dravet Syndrome
- PMID: 28490751
- PMCID: PMC5431801
- DOI: 10.1038/s41598-017-01851-9
Unexpected Efficacy of a Novel Sodium Channel Modulator in Dravet Syndrome
Abstract
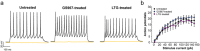
Dravet syndrome, an epileptic encephalopathy affecting children, largely results from heterozygous loss-of-function mutations in the brain voltage-gated sodium channel gene SCN1A. Heterozygous Scn1a knockout (Scn1a +/-) mice recapitulate the severe epilepsy phenotype of Dravet syndrome and are an accepted animal model. Because clinical observations suggest conventional sodium channel blocking antiepileptic drugs may worsen the disease, we predicted the phenotype of Scn1a +/- mice would be exacerbated by GS967, a potent, unconventional sodium channel blocker. Unexpectedly, GS967 significantly improved survival of Scn1a +/- mice and suppressed spontaneous seizures. By contrast, lamotrigine exacerbated the seizure phenotype. Electrophysiological recordings of acutely dissociated neurons revealed that chronic GS967-treatment had no impact on evoked action potential firing frequency of interneurons, but did suppress aberrant spontaneous firing of pyramidal neurons and was associated with significantly lower sodium current density. Lamotrigine had no effects on neuronal excitability of either neuron subtype. Additionally, chronically GS967-treated Scn1a +/- mice exhibited normalized pyramidal neuron sodium current density and reduced hippocampal NaV1.6 protein levels, whereas lamotrigine treatment had no effect on either pyramidal neuron sodium current or hippocampal NaV1.6 levels. Our findings demonstrate unexpected efficacy of a novel sodium channel blocker in Dravet syndrome and suggest a potential mechanism involving a secondary change in NaV1.6.
Conflict of interest statement
Dr. George received a research grant from Gilead Sciences, Inc., the manufacturer of GS967, which partially supported this work. Drs. Anderson, Hawkins, Thompson and Kearney declare no potential conflicts of interest.
Figures




References
-
- Dravet, C., Bureau, M., Guerrini, R., Giraud, N. & Toger, J. Epileptic Syndromes in Infancy, Childhood and Adolescence. Rogers, J., Bureau, M., Dravet, C., Dreifuss, F. E. & Wolf, P. (eds.), pp. 75–88 (John Libbey, London, 1992).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

