Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males
- PMID: 28490809
- PMCID: PMC5431852
- DOI: 10.1038/s41598-017-01937-4
Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males
Abstract
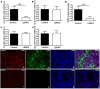
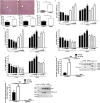
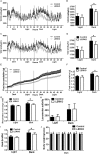
Impaired estrogens action is associated with features of the metabolic syndrome in animal models and humans. We sought to determine whether disruption of hepatic estrogens action in adult male mice could recapitulate aspects of the metabolic syndrome to understand the mechanistic basis for the phenotype. We found 17β-estradiol (E2) inhibited hepatic gluconeogenic genes such as phosphoenolpyruvate carboxykinase 1 (Pck-1) and glucose 6-phosphatase (G6Pase) and this effect was absent in mice lacking liver estrogen receptor α (Esr1) (LERKO mice). Male LERKO mice displayed elevated hepatic gluconeogenic activity and fasting hyperglycemia. We also observed increased liver lipid deposits and triglyceride levels in male LERKO mice, resulting from increased hepatic lipogenesis as reflected by increased mRNA levels of fatty acid synthase (Fas) and acetyl-CoA carboxylase (Acc1). ChIP assay demonstrated estradiol (E2) induced ESR1 binding to Pck-1, G6Pase, Fas and Acc1 promoters. Metabolic phenotyping demonstrated both basal metabolic rate and feeding were lower for the LERKO mice as compared to Controls. Furthermore, the respiratory exchange rate was significantly lower in LERKO mice than in Controls, suggesting an increase in lipid oxidation. Our data indicate that hepatic E2/ESR1 signaling plays a key role in the maintenance of gluconeogenesis and lipid metabolism in males.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

