Establishment of induced pluripotent stem cells from normal B cells and inducing AID expression in their differentiation into hematopoietic progenitor cells
- PMID: 28490810
- PMCID: PMC5431994
- DOI: 10.1038/s41598-017-01627-1
Establishment of induced pluripotent stem cells from normal B cells and inducing AID expression in their differentiation into hematopoietic progenitor cells
Abstract
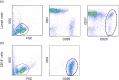
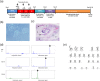
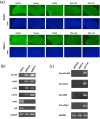
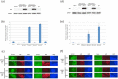
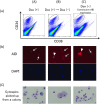
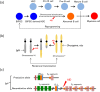
B cell derived induced pluripotent stem cells (BiPSCs) were recently established from peripheral blood B cells by the simultaneous transfection of Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc) and C/EBPα using a Sendai virus vector. Here, using a different method, we established BiPSCs with immunoglobulin heavy chain (IgH) gene rearrangement from normal B cells purified from lymph nodes. The critical points of our method are pre-stimulation of B cells with IL-21 and CD40-ligand (CD40L), followed by consecutive transfection of highly concentrated Yamanaka factors using a retroviral vector. Following each transfection the cells were centrifuged onto a retronectin coated plate and the activated by IL-4, IL-2, and CD40L. Furthermore, we established BiPSCs (BiPSC-A) in which activation-induced cytidine deaminase (AID) could be induced using the doxycycline-controlled. Both the parental BiPSC and BiPSC-A showed the capability of differentiating into hematopoietic progenitor cells (HPCs) based on confirmation of CD34 expression and colony-formation from CD34-positive cells. The findings that BiPSC-A can differentiate into HPCs suggest that there is a possibility that induction of AID expression would result in chromosomal translocations in the process of differentiation from BiPSCs, and therefore that these BiPSCs could be useful in elucidating the tumor origin of abnormal B cells in myelomagenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

