Quantitative and multiplexed detection for blood typing based on quantum dot-magnetic bead assay
- PMID: 28490874
- PMCID: PMC5413539
- DOI: 10.2147/IJN.S133247
Quantitative and multiplexed detection for blood typing based on quantum dot-magnetic bead assay
Abstract
Background: Accurate and reliable blood grouping is essential for safe blood transfusion. However, conventional methods are qualitative and use only single-antigen detection. We overcame these limitations by developing a simple, quantitative, and multiplexed detection method for blood grouping using quantum dots (QDs) and magnetic beads.
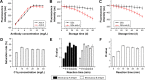
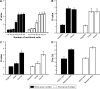
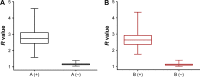
Methods: In the QD fluorescence assay (QFA), blood group A and B antigens were quantified using QD labeling and magnetic beads, and the blood groups were identified according to the R value (the value was calculated with the fluorescence intensity from dual QD labeling) of A and B antigens. The optimized performance of QFA was established by blood typing 791 clinical samples.
Results: Quantitative and multiplexed detection for blood group antigens can be completed within 35 min with more than 105 red blood cells. When conditions are optimized, the assay performance is satisfactory for weak samples. The coefficients of variation between and within days were less than 10% and the reproducibility was good. The ABO blood groups of 791 clinical samples were identified by QFA, and the accuracy obtained was 100% compared with the tube test. Receiver-operating characteristic curves revealed that the QFA has high sensitivity and specificity toward clinical samples, and the cutoff points of the R value of A and B antigens were 1.483 and 1.576, respectively.
Conclusion: In this study, we reported a novel quantitative and multiplexed method for the identification of ABO blood groups and presented an effective alternative for quantitative blood typing. This method can be used as an effective tool to improve blood typing and further guarantee clinical transfusion safety.
Keywords: blood group antigens; blood typing; fluorescence detection; magnetic beads; quantum dots.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Figueras-Aloy J, Rodríguez-Miguélez JM, Iriondo-Sanz M, Salvia-Roiges MD, Botet-Mussons F, Carbonell-Estrany X. Intravenous immunoglobulin and necrotizing enterocolitis in newborns with hemolytic disease. Pediatrics. 2010;125(1):139–144. - PubMed
-
- Zuppa AA, Cardiello V, Lai M, Cataldi L, D’Andrea V, Romagnoli C. ABO hemolytic disease of the fetus and newborn: an iatrogenic complication of heterologous assisted reproductive technology-induced pregnancy. Transfusion. 2010;50(10):2102–2104. - PubMed
-
- Bhat RY, Kumar PC. Sixth hour transcutaneous bilirubin predicting significant hyperbilirubinemia in ABO incompatible neonates. World J Pediatr. 2014;10(2):182–185. - PubMed
-
- Doyle B, Quigley J, Lambert M, et al. A correlation between severe haemolytic disease of the fetus and newborn and maternal ABO blood group. Transfus Med. 2014;24(4):239–243. - PubMed
-
- Yogev-Lifshitz M, Leibovitch L, Schushan-Eisen I, Taran C, Strauss T, Maayan-Metzger A. Indication of mild hemolytic reaction among preterm infants with ABO incompatibility. Pediatr Blood Cancer. 2016;63(6):1050–1053. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

