A novel injectable calcium phosphate-based nanocomposite for the augmentation of cannulated pedicle-screw fixation
- PMID: 28490878
- PMCID: PMC5414751
- DOI: 10.2147/IJN.S131962
A novel injectable calcium phosphate-based nanocomposite for the augmentation of cannulated pedicle-screw fixation
Abstract
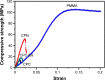
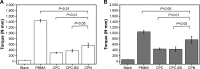
Polymethyl methacrylate (PMMA)-augmented cannulated pedicle-screw fixation has been routinely performed for the surgical treatment of lumbar degenerative diseases. Despite its satisfactory clinical outcomes and prevalence, problems and complications associated with high-strength, stiff, and nondegradable PMMA have largely hindered the long-term efficacy and safety of pedicle-screw fixation in osteoporotic patients. To meet the unmet need for better bone cement for cannulated pedicle-screw fixation, a new injectable and biodegradable nanocomposite that was the first of its kind was designed and developed in the present study. The calcium phosphate-based nanocomposite (CPN) exhibited better anti-pullout ability and similar fluidity and dispersing ability compared to clinically used PMMA, and outperformed conventional calcium phosphate cement (CPC) in all types of mechanical properties, injectability, and biodegradability. In term of axial pullout strength, the CPN-augmented cannulated screw reached the highest force of ~120 N, which was higher than that of PMMA (~100 N) and CPC (~95 N). The compressive strength of the CPN (50 MPa) was three times that of CPC, and the injectability of the CPN reached 95%. In vivo tests on rat femur revealed explicit biodegradation of the CPN and subsequent bone ingrowth after 8 weeks. The promising results for the CPN clearly suggest its potential for replacing PMMA in the application of cannulated pedicle-screw fixation and its worth of further study and development for clinical uses.
Keywords: biodegradable; calcium phosphate nanocomposite; injectable; lumbar degenerative disease; osteoporosis; pedicle screw.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








References
-
- Galbusera F, Volkheimer D, Reitmaier S, Berger-Roscher N, Kienle A, Wilke HJ. Pedicle screw loosening: a clinically relevant complication? Eur Spine J. 2015;24(5):1005–1016. - PubMed
-
- Bredow J, Boese CK, Werner CM, et al. Predictive validity of preoperative CT scans and the risk of pedicle screw loosening in spinal surgery. Arch Orthop Trauma Surg. 2016;136(8):1063–1067. - PubMed
-
- Padányi C, Misik F, Papp Z, et al. Treatment of osteoporotic vertebral compression fractures with PMMA-augmented pedicle screw fixation. Ideggyogyaszati Szemle. 2015;68(1–2):52–58. Hungarian. - PubMed
-
- Henroteaux A, Racaru T, Martin D, Scholtes F, Dubuisson A, Kaschten B. Polymethylmethacrylate (PMMA) augmentation of the pedicle screws in patients with fractures of osteoporotic bone. About our experience. conference abstract, Société Belge de Neurochirurgie, Mar 29, 2014. [Accessed March 26, 2017]. Available from: http://hdl.handle.net/2268/165163.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

