Patient and physician preferences for anticancer drugs for the treatment of metastatic colorectal cancer: a discrete-choice experiment
- PMID: 28490902
- PMCID: PMC5414575
- DOI: 10.2147/CMAR.S125245
Patient and physician preferences for anticancer drugs for the treatment of metastatic colorectal cancer: a discrete-choice experiment
Abstract
Objective: Many publications describe preferences for colorectal cancer (CRC) screening; however, few studies elicited preferences for anticancer-drug treatment for metastatic CRC (mCRC). This study was designed to elicit preferences and risk tolerance among patients and oncologists in the USA for anticancer drugs to treat mCRC.
Materials and methods: Patients aged 18 years or older with a self-reported diagnosis of mCRC and board-certified (or equivalent) oncologists who had treated patients with mCRC were recruited by two survey research companies from existing online patient panels in the USA. Additional oncologists were recruited from a list of US physicians. Patients and oncologists completed a discrete-choice experiment (DCE) survey. DCEs offer a systematic method of eliciting preferences and quantifying both the relative importance of treatment attributes and the tradeoffs respondents are willing to make among benefits and risks. Treatment attributes in the DCE were progression-free survival (PFS) and risks of severe papulopustular rash, serious hemorrhage, cardiopulmonary arrest, and gastrointestinal perforation. Patients' and physicians' maximum levels of acceptable treatment-related risks for two prespecified increases in efficacy were estimated.
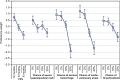
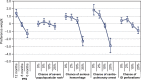
Results: A total of 127 patients and 150 oncologists completed the survey. Relative preferences for the treatment attributes in the study were mostly consistent with the expectation that better clinical outcomes were preferred over worse clinical outcomes. Risk tolerance varied between patients and physicians. On average, physicians were willing to tolerate higher risks than patients, although these differences were mostly not statistically significant. Post hoc latent-class analyses revealed that some patients and physicians were unwilling to forgo any efficacy to avoid toxicities, while others were willing to make such tradeoffs.
Conclusion: Differences in preferences between patients and physicians suggest that there is the potential for improvement in patients' well-being. Initiating or enhancing discussions about patient tolerance for toxicities, such as skin rash and gastrointestinal perforations, may help prescribe treatments that entail more appropriate benefit-risk tradeoffs.
Keywords: discrete-choice experiment; metastatic colorectal cancer; patient preferences; physician preferences; risk tolerance.
Conflict of interest statement
Disclosure Brett Hauber and Joshua Posner are employees of RTI Health Solutions, which received research funding from Genentech. Juan Marcos González was an employee of RTI Health Solutions at the time of the study. Nicolas Sommer and Sarika Ogale are employees of Genentech; Robert Morlock was an employee of Genentech at the time of the study. The authors report no other conflicts of interest in this work.
Figures

References
-
- American Cancer Society What are the key statistics about colorectal cancer. 2016. [Accessed February 7, 2016]. Available from: http://www.cancer.org/cancer/colonan-drectumcancer/detailedguide/colorec....
-
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680. - PubMed
-
- Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32(3):437–453. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

