Other non-surgical treatments for liver cancer
- PMID: 28490991
- PMCID: PMC5411904
- DOI: 10.1016/j.rpor.2017.02.007
Other non-surgical treatments for liver cancer
Abstract
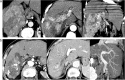
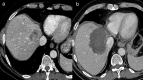
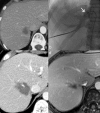
Interventional radiology plays a major role in the modern management of liver cancers, in primary hepatic malignancies or metastases and in palliative or curative situations. Radiological treatments are divided in two categories based on their approach: endovascular treatment and direct transcapsular access. Endovascular treatments include mainly three applications: transarterial chemoembolization (TACE), transarterial radioembolization (TARE) and portal vein embolization (PVE). TACE and TARE share an endovascular arterial approach, consisting of a selective catheterization of the hepatic artery or its branches. Subsequently, either a chemotherapy (TACE) or radioembolic (TARE) agent is injected in the target vessel to act on the tumor. PVE raises the volume of the future liver remnant in extended hepatectomy by embolizing a portal vein territory which results in hepatic regeneration. Direct transcapsular access treatments involve mainly three techniques: radiofrequency thermal ablation (RFA), microwave thermal ablation (MWA) and percutaneous ethanol injection (PEI). RFA and MWA procedures are almost identical, their clinical applications are similar. A probe is deployed directly into the tumor to generate heat and coagulation necrosis. PEI has known implications based on the chemical toxicity of intra-tumoral injection with highly concentrated alcohol by a thin needle.
Keywords: Microwave thermal ablation; Percutaneous ethanol injection; Portal vein embolization; Radiofrequency thermal ablation; Transarterial chemoembolization; Transarterial radioembolization.
Figures





References
-
- Wang P.M., Chung N.N., Hsu W.C., Chang F.L., Jang C.J., Scorsetti M. Stereotactic body radiation therapy in hepatocellular carcinoma: optimal treatment strategies based on liver segmentation and functional hepatic reserve. Rep Pract Oncol Radiother. 2015;20(November–December (6)):417–424. - PMC - PubMed
-
- Mondazzi L., Bottelli R., Brambilla G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19(May (5)):1115–1123. - PubMed
-
- Dumortier J., Chapuis F., Borson O. Unresectable hepatocellular carcinoma: survival and prognostic factors after lipiodol chemoembolisation in 89 patients. Dig Liver Dis. 2006;38(February (2)):125–133. - PubMed
-
- Tam K.Y., Leung K.C., Wang Y.X. Chemoembolization agents for cancer treatment. Eur J Pharm Sci. 2011;44(September (1–2)):1–10. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

