Change of Rin1 and Stathmin in the Animal Model of Traumatic Stresses
- PMID: 28491025
- PMCID: PMC5405079
- DOI: 10.3389/fnbeh.2017.00062
Change of Rin1 and Stathmin in the Animal Model of Traumatic Stresses
Abstract
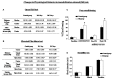
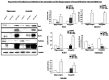
The molecular mechanism of fear memory is poorly understood. Therefore, the pathogenesis of post-traumatic stress disorder (PTSD), whose symptom presentation can enhance fear memory, remains largely unclear. Recent studies with knockout animals have reported that Rin1 and stathmin regulate fear memory. Rin1 inhibits acquisition and promotes memory extinction, whereas stathmin regulates innate and basal fear. The aim of our study was to examine changes in the expression of Rin1 and stathmin in different animal models of stress, particluarly traumatic stress. We used three animal traumatic stresses: single prolonged stress (SPS, which is a rodent model of PTSD), an immobilization-stress (IM) and a Loud sound stress (LSS), to examine the change and uniqueness in Rin1/stathmin expression. Behavioral tests of SPS rats demonstrated increased anxiety and contextual fear-conditioning. They showed decreased long-term potentiation (LTP), as well as decreased stathmin and increased Rin1 expression in the hippocampus and the amygdala. Expression of the stathmin effector, tubulin, and downstream molecules Rin1, Rab5, and Abl, appeared to increase. Rin1 and EphA4 were endogenously coexpressed in primary neurons after SPS stimulation. IM rats exhibited increased anxiety behavior and enhanced fear-conditioning to contextual and auditory stimuli. Similar changes in expression of Rin1/stathmin were observed in IM rats whereas no changes were observed in rats exposed to a loud sound. These data suggest that changes in expression of the Rin1 and stathmin genes may be involved in rodents with SPS and IM stresses, which provide valuable insight into fear memories under abnormal conditions, particularly in PTSD.
Keywords: Rin1; fear memory; post-traumatic disorder; single prolonged stress; stathmin; traumatic stress.
Figures








Similar articles
-
Stathmin Regulates Spatiotemporal Variation in the Memory Loop in Single-Prolonged Stress Rats.J Mol Neurosci. 2020 Apr;70(4):576-589. doi: 10.1007/s12031-019-01459-w. Epub 2020 Jan 13. J Mol Neurosci. 2020. PMID: 31933182
-
STAT3 induces amygdala apoptosis by regulating the expression of stathmin in the rat model of posttraumatic stress disorder.Folia Neuropathol. 2025;63(1):87-99. doi: 10.5114/fn.2024.140830. Folia Neuropathol. 2025. PMID: 40353381
-
Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model.Neuroscience. 2007 Aug 10;148(1):22-33. doi: 10.1016/j.neuroscience.2007.05.041. Epub 2007 Jul 17. Neuroscience. 2007. PMID: 17644267
-
Single prolonged stress: toward an animal model of posttraumatic stress disorder.Depress Anxiety. 2009;26(12):1110-7. doi: 10.1002/da.20629. Depress Anxiety. 2009. PMID: 19918929 Review.
-
[A novel therapeutic approach for the treatment of post-traumatic stress disorder (PTSD): enhancing the impaired extinction of fear memory].Nihon Shinkei Seishin Yakurigaku Zasshi. 2009 Jun;29(3):135-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2009. PMID: 19663264 Review. Japanese.
Cited by
-
Effects of Social Defeat Stress on Microtubule Regulating Proteins and Tubulin Polymerization.Clin Psychopharmacol Neurosci. 2024 Feb 29;22(1):129-138. doi: 10.9758/cpn.23.1077. Epub 2023 Aug 10. Clin Psychopharmacol Neurosci. 2024. PMID: 38247419 Free PMC article.
-
Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress.Brain Sci. 2019 Aug 26;9(9):215. doi: 10.3390/brainsci9090215. Brain Sci. 2019. PMID: 31454951 Free PMC article.
-
Myelination defects in the medial prefrontal cortex of Fkbp5 knockout mice.FASEB J. 2021 Feb;35(2):e21297. doi: 10.1096/fj.202001883R. FASEB J. 2021. PMID: 33410216 Free PMC article.
-
Traumatic Stress Produces Distinct Activations of GABAergic and Glutamatergic Neurons in Amygdala.Front Neurosci. 2018 Aug 21;12:387. doi: 10.3389/fnins.2018.00387. eCollection 2018. Front Neurosci. 2018. PMID: 30186100 Free PMC article.
-
Impact of Social Defeat Stress on DNA Methylation in Drd2, Nr3c1, and Stmn1 in Wild-type and Stmn1 Knock-out Mice.Clin Psychopharmacol Neurosci. 2022 Feb 28;20(1):51-60. doi: 10.9758/cpn.2022.20.1.51. Clin Psychopharmacol Neurosci. 2022. PMID: 35078948 Free PMC article.
References
-
- American Psychiatric Association (2013). The Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th Edn. Arlington, VA: American Psychiatric Association.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

