Proteomic Analysis Reveals Autophagy as Pro-Survival Pathway Elicited by Long-Term Exposure with 5-Azacitidine in High-Risk Myelodysplasia
- PMID: 28491035
- PMCID: PMC5405131
- DOI: 10.3389/fphar.2017.00204
Proteomic Analysis Reveals Autophagy as Pro-Survival Pathway Elicited by Long-Term Exposure with 5-Azacitidine in High-Risk Myelodysplasia
Abstract
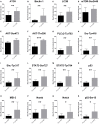
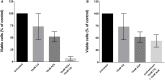
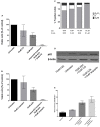
Azacytidine (5-AZA) is the standard first-choice treatment for high-risk myelodysplasia (MDS) patients. However, the clinical outcome for those patients who interrupt treatment or whose disease failed to respond is very poor. In order to identify the cellular pathways that are modified by long-term exposure to 5-AZA, we evaluated key proteins associated with the autophagy pathway by reverse-phase microarray (RPPA). Comparing bone marrow mononucleated cells (BMMCs) obtained from 20 newly-diagnosed patients and after four 5-AZA cycles we found an increased autophagy signaling. We then evaluated ex-vivo the effect of the combination of 5-AZA with autophagy inhibitors chloroquine (CQ) and leupeptin. Since 5-AZA and CQ showed synergism due to an increase of basal autophagy after 5-AZA exposure, we adopted a sequential treatment treating BMMCs with 5 μM 5-AZA for 72 h followed by 10 μM CQ for 24 h and found increased apoptosis, associated to a reduction of G2M phase and increase in G0-G1 phase. Long-term exposure to 5-AZA induced the reduction of the autophagic marker SQSTM1/p62, reversible by CQ or leupeptin exposure. In conclusion, we identified autophagy as a compensatory pathway occurring in MDS-BM after long-term exposure to 5-AZA and we provided evidences that a sequential treatment of 5-AZA followed by CQ could improve 5-AZA efficacy, providing novel insight for tailored therapy in MDS patients progressing after 5-AZA therapy.
Keywords: autophagy; azacytidine; myelodysplastic syndrome.
Figures
Similar articles
-
The Hedgehog pathway as targetable vulnerability with 5-azacytidine in myelodysplastic syndrome and acute myeloid leukemia.J Hematol Oncol. 2015 Oct 20;8:114. doi: 10.1186/s13045-015-0211-8. J Hematol Oncol. 2015. PMID: 26483188 Free PMC article.
-
Silencing HO-1 sensitizes SKM-1 cells to apoptosis induced by low concentration 5-azacytidine through enhancing p16 demethylation.Int J Oncol. 2015 Mar;46(3):1317-27. doi: 10.3892/ijo.2015.2835. Epub 2015 Jan 12. Int J Oncol. 2015. PMID: 25585641
-
A novel non-invasive monitoring assay of 5-azacitidine efficacy using global DNA methylation of peripheral blood in myelodysplastic syndrome.Drug Des Devel Ther. 2019 May 30;13:1821-1833. doi: 10.2147/DDDT.S195071. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31239639 Free PMC article.
-
Treatment of advanced myelodysplastic syndrome with demethylating agents: azacitidine.Semin Hematol. 2012 Oct;49(4):323-9. doi: 10.1053/j.seminhematol.2012.09.002. Semin Hematol. 2012. PMID: 23079062 Review.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.Health Technol Assess. 2010 May;14 Suppl 1:69-74. doi: 10.3310/hta14Suppl1/10. Health Technol Assess. 2010. PMID: 20507806 Review.
Cited by
-
Proteomics to predict relapse in patients with myelodysplastic neoplasms undergoing allogeneic hematopoietic cell transplantation.Biomark Res. 2024 Jan 25;12(1):10. doi: 10.1186/s40364-023-00550-0. Biomark Res. 2024. PMID: 38273355 Free PMC article.
-
Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets.Signal Transduct Target Ther. 2023 Jan 16;8(1):32. doi: 10.1038/s41392-022-01300-8. Signal Transduct Target Ther. 2023. PMID: 36646695 Free PMC article. Review.
-
Restoration of Autophagy and Apoptosis in Myelodysplastic Syndromes: The Effect of Azacitidine in Disease Pathogenesis.Curr Issues Mol Biol. 2025 Jul 4;47(7):520. doi: 10.3390/cimb47070520. Curr Issues Mol Biol. 2025. PMID: 40728989 Free PMC article.
-
Azacitidine induced lung injury: report and contemporary discussion on diagnosis and management.Front Oncol. 2024 Feb 9;14:1345492. doi: 10.3389/fonc.2024.1345492. eCollection 2024. Front Oncol. 2024. PMID: 38406809 Free PMC article. Review.
-
Small molecules re-establish neural cell fate of human fibroblasts via autophagy activation.In Vitro Cell Dev Biol Anim. 2019 Sep;55(8):622-632. doi: 10.1007/s11626-019-00381-0. Epub 2019 Jul 18. In Vitro Cell Dev Biol Anim. 2019. PMID: 31321620
References
-
- Bellodi C., Lidonnici M. R., Hamilton A., Helgason G. V., Soliera A. R., Ronchetti M., et al. . (2009). Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest. 119, 1109–1123. 10.1172/JCI35660 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

