Receptor-Mediated Melanoma Targeting with Radiolabeled α-Melanocyte-Stimulating Hormone: Relevance of the Net Charge of the Ligand
- PMID: 28491052
- PMCID: PMC5405074
- DOI: 10.3389/fendo.2017.00093
Receptor-Mediated Melanoma Targeting with Radiolabeled α-Melanocyte-Stimulating Hormone: Relevance of the Net Charge of the Ligand
Abstract
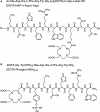
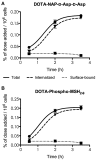
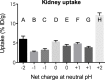
A majority of melanotic and amelanotic melanomas overexpress melanocortin type 1 receptors (MC1Rs) for α-melanocyte-stimulating hormone. Radiolabeled linear or cyclic analogs of α-MSH have a great potential as diagnostic or therapeutic tools for the management of malignant melanoma. Compounds such as [111In]DOTA-NAP-amide exhibit high affinity for the MC1R in vitro, good tumor uptake in vivo, but they may suffer from relatively high kidney uptake and retention in vivo. We have shown previously that the introduction of negative charges into radiolabeled DOTA-NAP-amide peptide analogs may enhance their excretion and reduce kidney retention. To address the question of where to place negative charges within the ligand, we have extended these studies by designing two novel peptides, Ac-Nle-Asp-His-d-Phe-Arg-Trp-Gly-Lys(DOTA)-d-Asp-d-Asp-OH (DOTA-NAP-d-Asp-d-Asp) with three negative charges at the C-terminal end (overall net charge of the molecule -2) and DOTA-Gly-Tyr(P)-Nle-Asp-His-d-Phe-Arg-Trp-NH2 (DOTA-Phospho-MSH2-9) with two negative charges in the N-terminal region (net charge -1). The former peptide showed markedly reduced receptor affinity and biological activity by >10-fold compared to DOTA-NAP-amide as reference compound, and the latter peptide displayed similar bioactivity and receptor affinity as the reference compound. The uptake by melanoma tumor tissue of [111In]DOTA-Phospho-MSH2-9 was 7.33 ± 0.47 %ID/g 4 h after injection, i.e., almost equally high as with [111In]DOTA-NAP-amide. The kidney retention was 2.68 ± 0.18 %ID/g 4 h after injection and hence 44% lower than that of [111In]DOTA-NAP-amide. Over an observation period from 4 to 48 h, the tumor-to-kidney ratio of [111In]DOTA-Phospho-MSH2-9 was 35% more favorable than that of the reference compound. In a comparison of DOTA-NAP-d-Asp-d-Asp, DOTA-Phospho-MSH2-9 and DOTA-NAP-amide with five previously published analogs of DOTA-NAP-amide that altogether cover a range of peptides with an overall net charge between +2 and -2, we now demonstrate that a net charge of -1, with the extra negative charges preferably placed in the N-terminal region, has led to the lowest kidney uptake and retention. Charges of +2 or -2 markedly increased kidney uptake and retention. In conclusion, the novel DOTA-Phospho-MSH2-9 may represent a new lead compound for negatively charged linear MC1R ligands that can be further developed into a clinically relevant melanoma targeting radiopeptide.
Keywords: kidney toxicity; melanoma; net charge; phosphopeptide; radiolabeled peptide; tissue distribution; tumor targeting; α-melanocyte-stimulating hormone.
Figures


Similar articles
-
Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake.J Nucl Med. 2005 May;46(5):887-95. J Nucl Med. 2005. PMID: 15872364
-
[NIe4,Asp5,d-Phe7, 67Ga/68Ga-1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-1,4,7,10-tetraacetic acid-Lys11]-α-MSH4-11.2007 Jul 30 [updated 2007 Nov 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Jul 30 [updated 2007 Nov 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641555 Free Books & Documents. Review.
-
Dimeric DOTA-alpha-melanocyte-stimulating hormone analogs: synthesis and in vivo characteristics of radiopeptides with high in vitro activity.J Recept Signal Transduct Res. 2007;27(5-6):383-409. doi: 10.1080/10799890701723528. J Recept Signal Transduct Res. 2007. PMID: 18097939
-
A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis.J Nucl Med. 2002 Dec;43(12):1699-706. J Nucl Med. 2002. PMID: 12468522
-
111In-DOTA-Re(Cys3,4,10,d-Phe7,Arg11)αMSH3-13.2007 Sep 1 [updated 2007 Dec 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Sep 1 [updated 2007 Dec 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641798 Free Books & Documents. Review.
Cited by
-
Optimizing the Safety and Efficacy of Bio-Radiopharmaceuticals for Cancer Therapy.Pharmaceutics. 2023 Apr 30;15(5):1378. doi: 10.3390/pharmaceutics15051378. Pharmaceutics. 2023. PMID: 37242621 Free PMC article.
-
Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands.Pharmaceuticals (Basel). 2024 Feb 16;17(2):256. doi: 10.3390/ph17020256. Pharmaceuticals (Basel). 2024. PMID: 38399470 Free PMC article. Review.
-
Trends in nanobody radiotheranostics.Eur J Nucl Med Mol Imaging. 2025 May;52(6):2225-2238. doi: 10.1007/s00259-025-07077-6. Epub 2025 Jan 13. Eur J Nucl Med Mol Imaging. 2025. PMID: 39800806 Review.
-
212Pb in targeted radionuclide therapy: a review.EJNMMI Radiopharm Chem. 2025 Jul 1;10(1):34. doi: 10.1186/s41181-025-00362-7. EJNMMI Radiopharm Chem. 2025. PMID: 40591218 Free PMC article. Review.
-
An Efficient Method for Labeling Single Domain Antibody Fragments with 18F Using Tetrazine- Trans-Cyclooctene Ligation and a Renal Brush Border Enzyme-Cleavable Linker.Bioconjug Chem. 2018 Dec 19;29(12):4090-4103. doi: 10.1021/acs.bioconjchem.8b00699. Epub 2018 Nov 14. Bioconjug Chem. 2018. PMID: 30384599 Free PMC article.
References
-
- Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, et al. Characterization of receptors for α-melanocyte-stimulating hormone on human melanoma cells. Cancer Res (1989) 49:6352–8. - PubMed
-
- Loir B, Pérez Sánchez C, Ghanem G, Lozano JA, García-Borrón JC, Jiménez-Cervantes G. Expression of the MC1 receptor gene in normal and malignant human melanocytes. A semiquantitative RT-PCR study. Cell Mol Biol (1999) 45:1083–92. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

