ISA-2011B, a Phosphatidylinositol 4-Phosphate 5-Kinase α Inhibitor, Impairs CD28-Dependent Costimulatory and Pro-inflammatory Signals in Human T Lymphocytes
- PMID: 28491063
- PMCID: PMC5405084
- DOI: 10.3389/fimmu.2017.00502
ISA-2011B, a Phosphatidylinositol 4-Phosphate 5-Kinase α Inhibitor, Impairs CD28-Dependent Costimulatory and Pro-inflammatory Signals in Human T Lymphocytes
Abstract
Phosphatidylinositol 4,5-biphosphate (PIP2) is a membrane phospholipid that controls the activity of several proteins regulating cytoskeleton reorganization, cytokine gene expression, T cell survival, proliferation, and differentiation. Phosphatidylinositol 4-phosphate 5-kinases (PIP5Ks) are the main enzymes involved in PIP2 biosynthesis by phosphorylating phosphatidylinositol 4-monophosphate (PI4P) at the D5 position of the inositol ring. In human T lymphocytes, we recently found that CD28 costimulatory molecule is pivotal for PIP2 turnover by recruiting and activating PIP5Kα. We also found that PIP5Kα is the main regulator of both CD28 costimulatory signals integrating those delivered by TCR as well as CD28 autonomous signals regulating the expression of pro-inflammatory genes. Given emerging studies linking alterations of PIP2 metabolism to immune-based diseases, PIP5Kα may represent a promising target to modulate immunity and inflammation. Herewith, we characterized a recently discovered inhibitor of PIP5Kα, ISA-2011B, for its inhibitory effects on T lymphocyte functions. We found that the inhibition of PIP5Kα lipid-kinase activity by ISA-2011B significantly impaired CD28 costimulatory signals necessary for TCR-mediated Ca2+ influx, NF-AT transcriptional activity, and IL-2 gene expression as well as CD28 autonomous signals regulating the activation of NF-κB and the transcription of pro-inflammatory cytokine and chemokine genes. Moreover, our data on the inhibitory effects of ISA-2011B on CD28-mediated upregulation of inflammatory cytokines related to Th17 cell phenotype in type 1 diabetes patients suggest ISA-2011B as a promising anti-inflammatory drug.
Keywords: CD28 co-stimulation; PIP5K; T lymphocytes; T1D; pro-inflammatory cytokines.
Figures
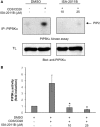

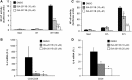

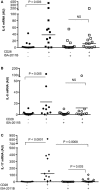
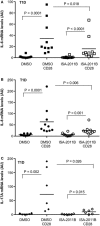
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

