Optimal Solubility of Diclofenac β-Cyclodextrin in Combination with Local Anaesthetics for Mesotherapy Applications
- PMID: 28491114
- PMCID: PMC5402241
- DOI: 10.1155/2017/8321325
Optimal Solubility of Diclofenac β-Cyclodextrin in Combination with Local Anaesthetics for Mesotherapy Applications
Abstract
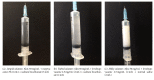
Because of low injection volume, the recently marketed injectable solution of diclofenac in complex with β-cyclodextrin (Akis®, IBSA Farmaceutici Italia) is an ideal candidate for mesotherapy applications. In this study, we investigated the solubility of Akis, 25 and 50 mg/kg, in combination with various local anaesthetics (lidocaine, mepivacaine, bupivacaine, levobupivacaine, and ropivacaine) at different concentrations in aqueous vehicles (normal saline, sterile water, or bicarbonate). Final injection mixtures were classified as limpid, turbid, or milky at visual analysis under standardized conditions. We found that (i) the use of sterile water for injections or normal saline as vehicles to dilute Akis in combination with whatever local anaesthetic normally results in milky solutions and therefore is not recommended; (ii) using bicarbonate, optimal solubility was obtained combining Akis with lidocaine, both 1 and 2%, or mepivacaine, both 1 and 2%, whereas solutions were turbid in combination with bupivacaine, levobupivacaine, or ropivacaine. Thus, we recommend that Akis is used in combination with lidocaine or mepivacaine in a bicarbonate vehicle.
Figures


References
-
- Mammucari M., Gatti A., Maggiori S., Bartoletti C. A., Sabato A. F. Mesotherapy, definition, rationale and clinical role: a consensus report from the Italian Society of Mesotherapy. European Review for Medical and Pharmacological Sciences. 2011;15(6):682–694. - PubMed
-
- Zeitlinger M., Rusca A., Oraha A. Z., Gugliotta B., Müller M., Ducharme M. P. Pharmacokinetics of a new diclofenac sodium formulation developed for subcutaneous and intramuscular administration. International Journal of Clinical Pharmacology and Therapeutics. 2012;50(6):383–390. doi: 10.5414/CP201600. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

