Outflow tract septation and the aortic arch system in reptiles: lessons for understanding the mammalian heart
- PMID: 28491275
- PMCID: PMC5424407
- DOI: 10.1186/s13227-017-0072-z
Outflow tract septation and the aortic arch system in reptiles: lessons for understanding the mammalian heart
Abstract
Background: Cardiac outflow tract patterning and cell contribution are studied using an evo-devo approach to reveal insight into the development of aorto-pulmonary septation.
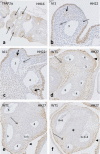
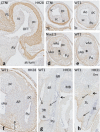
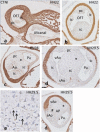
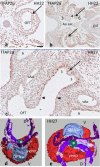
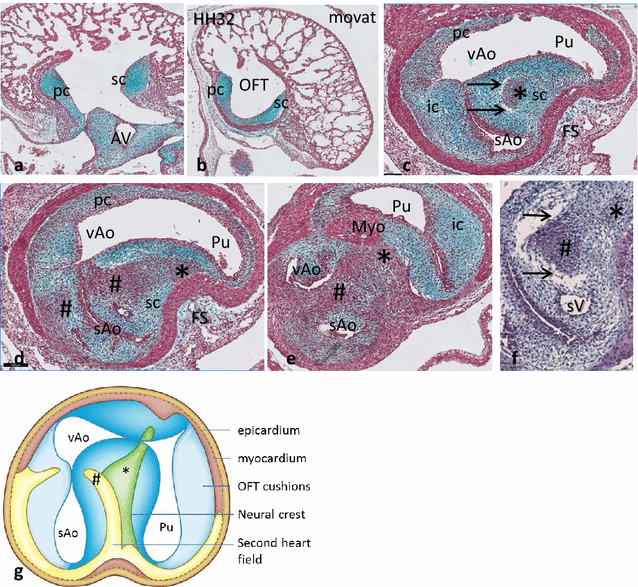
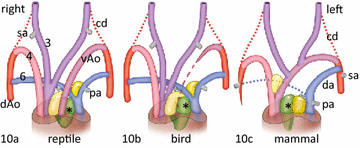
Results: We studied embryonic stages of reptile hearts (lizard, turtle and crocodile) and compared these to avian and mammalian development. Immunohistochemistry allowed us to indicate where the essential cell components in the outflow tract and aortic sac were deployed, more specifically endocardial, neural crest and second heart field cells. The neural crest-derived aorto-pulmonary septum separates the pulmonary trunk from both aortae in reptiles, presenting with a left visceral and a right systemic aorta arising from the unseptated ventricle. Second heart field-derived cells function as flow dividers between both aortae and between the two pulmonary arteries. In birds, the left visceral aorta disappears early in development, while the right systemic aorta persists. This leads to a fusion of the aorto-pulmonary septum and the aortic flow divider (second heart field population) forming an avian aorto-pulmonary septal complex. In mammals, there is also a second heart field-derived aortic flow divider, albeit at a more distal site, while the aorto-pulmonary septum separates the aortic trunk from the pulmonary trunk. As in birds there is fusion with second heart field-derived cells albeit from the pulmonary flow divider as the right 6th pharyngeal arch artery disappears, resulting in a mammalian aorto-pulmonary septal complex. In crocodiles, birds and mammals, the main septal and parietal endocardial cushions receive neural crest cells that are functional in fusion and myocardialization of the outflow tract septum. Longer-lasting septation in crocodiles demonstrates a heterochrony in development. In other reptiles with no indication of incursion of neural crest cells, there is either no myocardialized outflow tract septum (lizard) or it is vestigial (turtle). Crocodiles are unique in bearing a central shunt, the foramen of Panizza, between the roots of both aortae. Finally, the soft-shell turtle investigated here exhibits a spongy histology of the developing carotid arteries supposedly related to regulation of blood flow during pharyngeal excretion in this species.
Conclusions: This is the first time that is shown that an interplay of second heart field-derived flow dividers with a neural crest-derived cell population is a variable but common, denominator across all species studied for vascular patterning and outflow tract septation. The observed differences in normal development of reptiles may have impact on the understanding of development of human congenital outflow tract malformations.
Keywords: Aorto-pulmonary septation; Bird; Cardiac development; Crocodile; Flow divider; Neural crest; Outflow tract cushions; Reptiles; Second heart field.
Figures

Similar articles
-
Ventricular Septation and Outflow Tract Development in Crocodilians Result in Two Aortas with Bicuspid Semilunar Valves.J Cardiovasc Dev Dis. 2021 Oct 15;8(10):132. doi: 10.3390/jcdd8100132. J Cardiovasc Dev Dis. 2021. PMID: 34677201 Free PMC article.
-
Dual role for neural crest cells during outflow tract septation in the neural crest-deficient mutant Splotch(2H).J Anat. 2009 Feb;214(2):245-57. doi: 10.1111/j.1469-7580.2008.01028.x. J Anat. 2009. PMID: 19207986 Free PMC article.
-
Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure.Dev Biol. 1998 Apr 15;196(2):129-44. doi: 10.1006/dbio.1998.8860. Dev Biol. 1998. PMID: 9576827
-
Evolutionary and developmental origins of the cardiac neural crest: building a divided outflow tract.Birth Defects Res C Embryo Today. 2014 Sep;102(3):309-23. doi: 10.1002/bdrc.21076. Epub 2014 Sep 16. Birth Defects Res C Embryo Today. 2014. PMID: 25227322 Free PMC article. Review.
-
Cardiac Neural Crest Cells: Their Rhombomeric Specification, Migration, and Association with Heart and Great Vessel Anomalies.Cell Mol Neurobiol. 2021 Apr;41(3):403-429. doi: 10.1007/s10571-020-00863-w. Epub 2020 May 13. Cell Mol Neurobiol. 2021. PMID: 32405705 Free PMC article. Review.
Cited by
-
An Appreciation of Anatomy in the Molecular World.J Cardiovasc Dev Dis. 2020 Oct 15;7(4):44. doi: 10.3390/jcdd7040044. J Cardiovasc Dev Dis. 2020. PMID: 33076272 Free PMC article. Review.
-
Comparative analysis of avian hearts provides little evidence for variation among species with acquired endothermy.J Morphol. 2019 Mar;280(3):395-410. doi: 10.1002/jmor.20952. Epub 2019 Jan 22. J Morphol. 2019. PMID: 30667083 Free PMC article.
-
Morphogenetic processes in the development and evolution of the arteries of the pharyngeal arches: their relations to congenital cardiovascular malformations.Front Cell Dev Biol. 2023 Oct 12;11:1259175. doi: 10.3389/fcell.2023.1259175. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900278 Free PMC article. Review.
-
Reptiles as a Model System to Study Heart Development.Cold Spring Harb Perspect Biol. 2020 May 1;12(5):a037226. doi: 10.1101/cshperspect.a037226. Cold Spring Harb Perspect Biol. 2020. PMID: 31712265 Free PMC article. Review.
-
Clinical-pathological correlations of BAV and the attendant thoracic aortopathies. Part 2: Pluridisciplinary perspective on their genetic and molecular origins.J Mol Cell Cardiol. 2019 Aug;133:233-246. doi: 10.1016/j.yjmcc.2019.05.022. Epub 2019 Jun 6. J Mol Cell Cardiol. 2019. PMID: 31175858 Free PMC article. Review.
References
-
- Baardman ME, Zwier MV, Wisse LJ, Gittenberger-de Groot AC, Kerstjens-Frederikse WS, Hofstra RM, et al. Common arterial trunk and ventricular non-compaction in Lrp2 knockout mice indicate a crucial role of LRP2 in cardiac development. Dis Models Mech. 2016;9:413–425. doi: 10.1242/dmm.022053. - DOI - PMC - PubMed
-
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

