Push-pull dioxaborine as fluorescent molecular rotor: far-red fluorogenic probe for ligand-receptor interactions
- PMID: 28491320
- PMCID: PMC5421572
- DOI: 10.1039/C5TC03411F
Push-pull dioxaborine as fluorescent molecular rotor: far-red fluorogenic probe for ligand-receptor interactions
Abstract
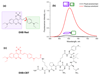
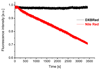
Fluorescent solvatochromic dyes and molecular rotors increase their popularity as fluorogenic probes for background-free detection of biomolecules in cellulo in no-wash conditions. Here, we introduce a push-pull boron-containing (dioxaborine) dye that presents unique spectroscopic behavior combining solvatochromism and molecular rotor properties. Indeed, in organic solvents, it shows strong red shifts in the absorption and fluorescence spectra upon increase in solvent polarity, typical for push-pull dyes. On the other hand, in polar solvents, where it probably undergoes Twisted Intramolecular Charge Transfer (TICT), the dye displays strong dependence of its quantum yield on solvent viscosity, in accordance to Förster-Hoffmann equation. In comparison to solvatochromic and molecular rotor dyes, dioxaborine derivative shows exceptional extinction coefficient (120,000 M-1 cm-1), high fluorescence quantum yields and red/far-red operating spectral range. It also displays much higher photostability in apolar media as compared to Nile Red, a fluorogenic dye of similar color. Its reactive carboxy derivative has been successfully grafted to carbetocin, a ligand of the oxytocin G protein-coupled receptor. This conjugate exhibits >1000-fold turn on between apolar 1,4-dioxane and water. It targets specifically the oxytocin receptor at the cell surface, which enables receptor imaging with excellent signal-to-background ratio (>130). We believe that presented push-pull dioxaborine dye opens a new page in the development of fluorogenic probes for bioimaging applications.
Figures
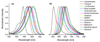


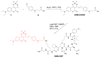

Similar articles
-
Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications.Acc Chem Res. 2017 Feb 21;50(2):366-375. doi: 10.1021/acs.accounts.6b00517. Epub 2017 Jan 9. Acc Chem Res. 2017. PMID: 28067047
-
Fluorogenic squaraine dimers with polarity-sensitive folding as bright far-red probes for background-free bioimaging.J Am Chem Soc. 2015 Jan 14;137(1):405-12. doi: 10.1021/ja5111267. Epub 2014 Dec 29. J Am Chem Soc. 2015. PMID: 25506627
-
Isomer-Specific Solvatochromic and Molecular Rotor Properties of ESIPT-Active Push-Pull Fluorescent Chalcone Dyes.J Phys Chem A. 2023 Oct 12;127(40):8365-8373. doi: 10.1021/acs.jpca.3c04903. Epub 2023 Sep 29. J Phys Chem A. 2023. PMID: 37773491
-
Push-Pull Fluorescent Dyes with Trifluoroacetyl Acceptor for High-Fidelity Sensing of Polarity and Heterogeneity of Lipid Droplets.Anal Chem. 2024 Aug 13;96(32):13242-13251. doi: 10.1021/acs.analchem.4c02322. Epub 2024 Jul 31. Anal Chem. 2024. PMID: 39083638
-
Recent advances in dioxaborine-based fluorescent materials for bioimaging applications.Mater Horiz. 2021 Feb 1;8(2):501-514. doi: 10.1039/d0mh01186j. Epub 2020 Nov 16. Mater Horiz. 2021. PMID: 34821266 Review.
Cited by
-
A fluorescent molecular rotor for the in situ imaging of latent fingerprints.RSC Adv. 2022 Nov 22;12(51):33180-33186. doi: 10.1039/d2ra06728e. eCollection 2022 Nov 15. RSC Adv. 2022. PMID: 36425194 Free PMC article.
-
Labelling primary immune cells using bright blue fluorescent nanoparticles.Biomater Sci. 2020 Mar 31;8(7):1897-1909. doi: 10.1039/c9bm01572h. Biomater Sci. 2020. PMID: 32026891 Free PMC article.
-
Multi-dimensional super-resolution imaging enables surface hydrophobicity mapping.Nat Commun. 2016 Dec 8;7:13544. doi: 10.1038/ncomms13544. Nat Commun. 2016. PMID: 27929085 Free PMC article.
-
Probing Liquid-Ordered and Disordered Phases in Lipid Model Membranes: A Combined Theoretical and Spectroscopic Study of a Fluorescent Molecular Rotor.J Phys Chem B. 2022 Jan 20;126(2):480-491. doi: 10.1021/acs.jpcb.1c08324. Epub 2022 Jan 10. J Phys Chem B. 2022. PMID: 35001625 Free PMC article.
-
Oxygen Sensing Difluoroboron β-Diketonate Polylactide Materials with Tunable Dynamic Ranges for Wound Imaging.ACS Sens. 2016 Nov 23;1(11):1366-1373. doi: 10.1021/acssensors.6b00533. Epub 2016 Nov 14. ACS Sens. 2016. PMID: 28042606 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
