PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3
- PMID: 28492231
- PMCID: PMC5437280
- DOI: 10.1038/ncomms15236
PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3
Abstract
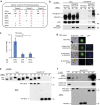
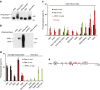
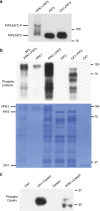
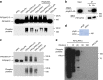
Upon light-induced nuclear translocation, phytochrome (phy) sensory photoreceptors interact with, and induce rapid phosphorylation and consequent ubiquitin-mediated degradation of, transcription factors, called PIFs, thereby regulating target gene expression and plant development. Nevertheless, the biochemical mechanism of phy-induced PIF phosphorylation has remained ill-defined. Here we identify a family of nuclear protein kinases, designated Photoregulatory Protein Kinases (PPK1-4; formerly called MUT9-Like Kinases (MLKs)), that interact with PIF3 and phyB in a light-induced manner in vivo. Genetic analyses demonstrate that the PPKs are collectively necessary for the normal light-induced phosphorylation and degradation of PIF3. PPK1 directly phosphorylates PIF3 in vitro, with a phosphosite pattern that strongly mimics the light-induced pattern in vivo. These data establish that the PPKs are directly involved in catalysing the photoactivated-phy-induced phosphorylation of PIF3 in vivo, and thereby are critical components of a transcriptionally centred signalling hub that pleiotropically regulates plant growth and development in response to multiple signalling pathways.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Ni M., Tepperman J. M. & Quail P. H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

