Tau association with synaptic vesicles causes presynaptic dysfunction
- PMID: 28492240
- PMCID: PMC5437271
- DOI: 10.1038/ncomms15295
Tau association with synaptic vesicles causes presynaptic dysfunction
Abstract
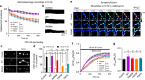
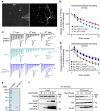
Tau is implicated in more than 20 neurodegenerative diseases, including Alzheimer's disease. Under pathological conditions, Tau dissociates from axonal microtubules and missorts to pre- and postsynaptic terminals. Patients suffer from early synaptic dysfunction prior to Tau aggregate formation, but the underlying mechanism is unclear. Here we show that pathogenic Tau binds to synaptic vesicles via its N-terminal domain and interferes with presynaptic functions, including synaptic vesicle mobility and release rate, lowering neurotransmission in fly and rat neurons. Pathological Tau mutants lacking the vesicle binding domain still localize to the presynaptic compartment but do not impair synaptic function in fly neurons. Moreover, an exogenously applied membrane-permeable peptide that competes for Tau-vesicle binding suppresses Tau-induced synaptic toxicity in rat neurons. Our work uncovers a presynaptic role of Tau that may be part of the early pathology in various Tauopathies and could be exploited therapeutically.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Ballatore C., Lee V. M. & Trojanowski J. Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007). - PubMed
-
- Wang Y. & Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 22–35 (2016). - PubMed
-
- Kwok J. B. et al.. Tau haplotypes regulate transcription and are associated with Parkinson's disease. Ann. Neurol. 55, 329–334 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

