Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors
- PMID: 28492341
- PMCID: PMC5669348
- DOI: 10.1177/0271678X17707398
Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors
Abstract
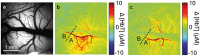
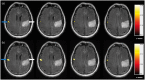
Functional magnetic resonance imaging (fMRI) serves as a critical tool for presurgical mapping of eloquent cortex and changes in neurological function in patients diagnosed with brain tumors. However, the blood-oxygen-level-dependent (BOLD) contrast mechanism underlying fMRI assumes that neurovascular coupling remains intact during brain tumor progression, and that measured changes in cerebral blood flow (CBF) are correlated with neuronal function. Recent preclinical and clinical studies have demonstrated that even low-grade brain tumors can exhibit neurovascular uncoupling (NVU), which can confound interpretation of fMRI data. Therefore, to avoid neurosurgical complications, it is crucial to understand the biophysical basis of NVU and its impact on fMRI. Here we review the physiology of the neurovascular unit, how it is remodeled, and functionally altered by brain cancer cells. We first discuss the latest findings about the components of the neurovascular unit. Next, we synthesize results from preclinical and clinical studies to illustrate how brain tumor induced NVU affects fMRI data interpretation. We examine advances in functional imaging methods that permit the clinical evaluation of brain tumors with NVU. Finally, we discuss how the suppression of anomalous tumor blood vessel formation with antiangiogenic therapies can "normalize" the brain tumor vasculature, and potentially restore neurovascular coupling.
Keywords: Neurovascular; angiogenesis; cancer; cooption; coupling; functional magnetic resonance imaging.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

