Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling
- PMID: 28492488
- PMCID: PMC5454943
- DOI: 10.3390/ijms18051031
Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling
Abstract
Background: Maintenance of brain circulation during shock is sufficient to prevent subcortical injury but the cerebral cortex is not spared. This suggests area-specific regulation of cerebral blood flow (CBF) during hemorrhage.
Methods: Cortical and subcortical CBF were continuously measured during blood loss (≤50%) and subsequent reperfusion using laser Doppler flowmetry. Blood gases, mean arterial blood pressure (MABP), heart rate and renal blood flow were also monitored. Urapidil was used for α1A-adrenergic receptor blockade in dosages, which did not modify the MABP-response to blood loss. Western blot and quantitative reverse transcription polymerase chain reactions were used to determine adrenergic receptor expression in brain arterioles.
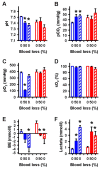
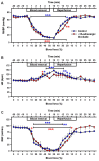
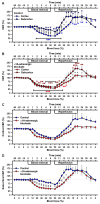
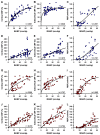
Results: During hypovolemia subcortical CBF was maintained at 81 ± 6% of baseline, whereas cortical CBF decreased to 40 ± 4% (p < 0.001). Reperfusion led to peak CBFs of about 70% above baseline in both brain regions. α1A-Adrenergic blockade massively reduced subcortical CBF during hemorrhage and reperfusion, and prevented hyperperfusion during reperfusion in the cortex. α1A-mRNA expression was significantly higher in the cortex, whereas α1D-mRNA expression was higher in the subcortex (p < 0.001).
Conclusions: α1-Adrenergic receptors are critical for perfusion redistribution: activity of the α1A-receptor subtype is a prerequisite for redistribution of CBF, whereas the α1D-receptor subtype may determine the magnitude of redistribution responses.
Keywords: adrenergic regulation; alpha-adrenergic; cerebral blood flow; cerebral hemodynamics; cerebrovasvular disease; head trauma; neurodegenerative disease; resuscitation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Underlying mechanism of subcortical brain protection during hypoxia and reoxygenation in a sheep model - Influence of α1-adrenergic signalling.PLoS One. 2018 May 29;13(5):e0196363. doi: 10.1371/journal.pone.0196363. eCollection 2018. PLoS One. 2018. PMID: 29813077 Free PMC article.
-
Altered Cerebral Blood Flow and Potential Neuroprotective Effect of Human Relaxin-2 (Serelaxin) During Hypoxia or Severe Hypovolemia in a Sheep Model.Int J Mol Sci. 2020 Feb 27;21(5):1632. doi: 10.3390/ijms21051632. Int J Mol Sci. 2020. PMID: 32120997 Free PMC article.
-
Relationship of cerebral blood flow to cardiac output, mean arterial pressure, blood volume, and alpha and beta blockade in cats.J Neurosurg. 1980 Jun;52(6):745-54. doi: 10.3171/jns.1980.52.6.0745. J Neurosurg. 1980. PMID: 6103920
-
Monitoring hemodynamic and metabolic alterations during severe hemorrhagic shock in rat brains.Acad Radiol. 2014 Feb;21(2):175-84. doi: 10.1016/j.acra.2013.11.017. Acad Radiol. 2014. PMID: 24439331 Review.
-
Possible role of alpha-adrenoceptor subtypes in acute migraine therapy.Cephalalgia. 2003 May;23(4):245-57. doi: 10.1046/j.1468-2982.2003.00547.x. Cephalalgia. 2003. PMID: 12716341 Review.
Cited by
-
Underlying mechanism of subcortical brain protection during hypoxia and reoxygenation in a sheep model - Influence of α1-adrenergic signalling.PLoS One. 2018 May 29;13(5):e0196363. doi: 10.1371/journal.pone.0196363. eCollection 2018. PLoS One. 2018. PMID: 29813077 Free PMC article.
-
Insight into Crosstalk between Ferroptosis and Necroptosis: Novel Therapeutics in Ischemic Stroke.Oxid Med Cell Longev. 2021 Jun 25;2021:9991001. doi: 10.1155/2021/9991001. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34257829 Free PMC article. Review.
-
Brain Histology and Immunohistochemistry After Resuscitation From Hemorrhagic Shock in Swine With Pre-Existing Atherosclerosis and Sodium Thiosulfate (Na2S2O3) Treatment.Front Med (Lausanne). 2022 Jun 30;9:925433. doi: 10.3389/fmed.2022.925433. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35847799 Free PMC article.
-
Altered Cerebral Blood Flow and Potential Neuroprotective Effect of Human Relaxin-2 (Serelaxin) During Hypoxia or Severe Hypovolemia in a Sheep Model.Int J Mol Sci. 2020 Feb 27;21(5):1632. doi: 10.3390/ijms21051632. Int J Mol Sci. 2020. PMID: 32120997 Free PMC article.
-
Sleep in the Supine Position during Pregnancy Is Associated with Fetal Cerebral Redistribution.J Clin Med. 2020 Jun 7;9(6):1773. doi: 10.3390/jcm9061773. J Clin Med. 2020. PMID: 32517385 Free PMC article.
References
-
- Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. - DOI - PubMed
-
- Schiffner R., Antonow-Schlorke I., Mueller T., Frasch M., Rupprecht S., Schubert H., Schwab M. Potential importance of alpha-receptors for distribution of cerebral blood flow (CBF) during umbilical cord occlusion (UCO) in fetal sheep-effects of betamethasone (BM) Reprod. Sci. 2010;17:300.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

